Question: Complete the K2 expression for HCO3 in an aqueous solution. K2 = 4.69 10-1 = Kal = 4.45 x 10-7 [HCO3] [H3O+] = [CO3-]
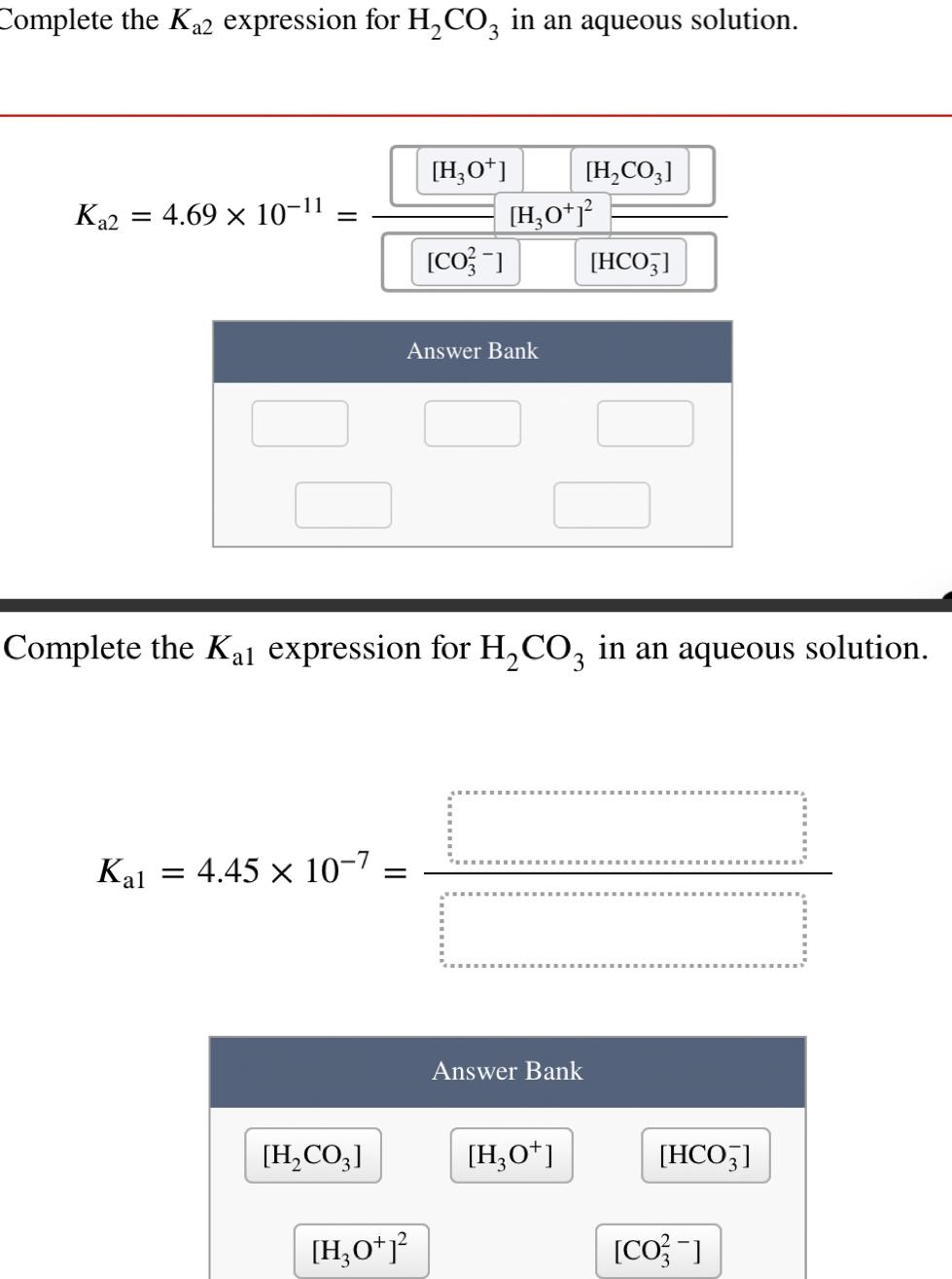
Complete the K2 expression for HCO3 in an aqueous solution. K2 = 4.69 10-1 = Kal = 4.45 x 10-7 [HCO3] [H3O+] = [CO3-] Answer Bank [HO+] Complete the Kal expression for HCO3 in an aqueous solution. [H0+1 [HCO3] Answer Bank [H3O+] [HCO3] [HCO3] [CO3-]
Step by Step Solution
3.49 Rating (156 Votes )
There are 3 Steps involved in it
Answer H Coz at H0 1 1 Kal 445157 1st ionizati... View full answer

Get step-by-step solutions from verified subject matter experts


