Question: Complete the table below: begin{tabular}{|l|c|l|} hline MANGANESE & Oxidation state of Mn & Color hline MnO4 & 7 & furple hline MnO2 &
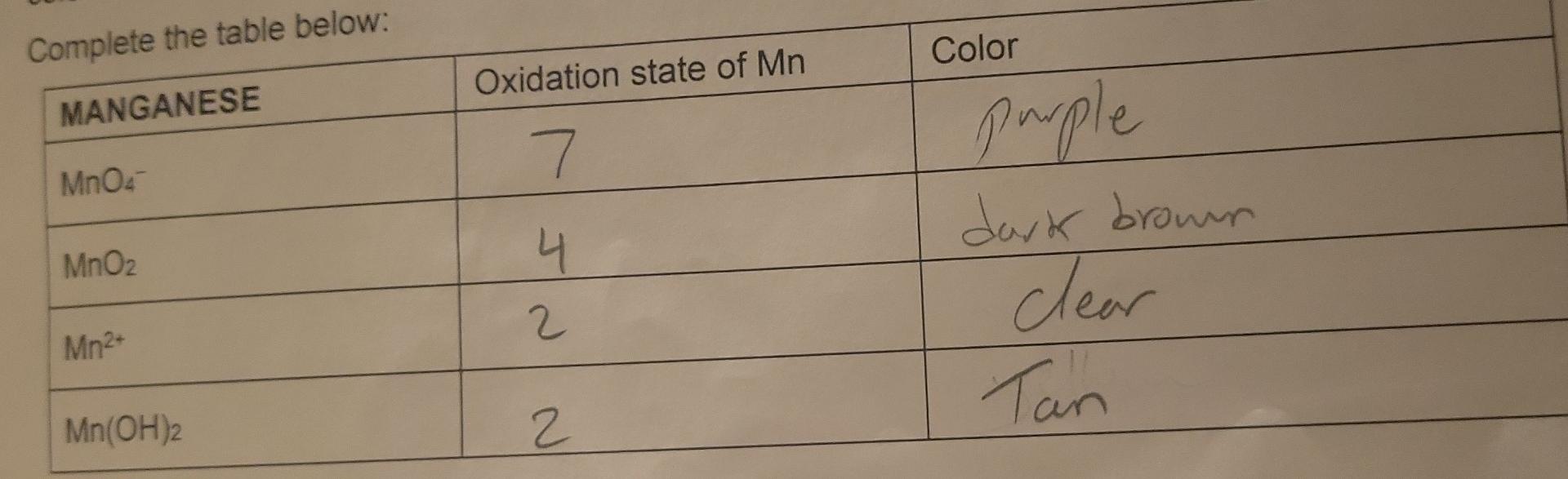

Complete the table below: \begin{tabular}{|l|c|l|} \hline MANGANESE & Oxidation state of Mn & Color \\ \hline MnO4 & 7 & furple \\ \hline MnO2 & 4 & dark brown \\ \hline Mn2+ & 2 & Clear \\ \hline Mn(OH)2 & 2 & Tan \\ \hline \end{tabular} Day 2: IDENTIFICATION OF OXIDATION-REDUCTION PRODUCTS UNDER VARYING CONDITIONS i. Basic conditions: Add 2mL of sodium hydroxide to 1mL of potassium permanganate. Then add hydrogen peroxide until the color change is complete. What color is the manganese product? dark brown Using this color, identify the manganese product. What is its formula? HnO2 What evidence identifies the product from hydrogen peroxide? pot black indicate the product is Complete the redox reaction and balance it under basic conditions (include phases for the final equation): MnO4+H2O2 ii. Neutral conditions: Add hydrogen peroxide directly to 1mL of potassium permanganate until the color change is complete. What color is the manganese product? Dawh Brown product. What is its formula? MnO ? What evidence identifies the product from hydrogen peroxide?pht smadl Brownspu indicate the product is Complete the redox reaction and balance it under neutral conditions (include phases for the final equation): MnO4+H2O2 iii. Acidic conditions Add 3mL of sulfuric acid to 1mL of potassium permanganate. Then add hydrogen peroxide until the color change is complete. What color is the manganese product? Clear Using this color, identify the manganese product. What is its formula? Mn2+ What evidence identifies the product from hydrogen peroxide? (evidence)bwbbles indicate the product is Complete the redox reaction and balance it under acidic conditions (include phases for the final equation): MnO4+H2O2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


