Question: Complete the table below for calculating the molar mass of the ionic compound cobalt(III) iodide. Molar mass of ion Mass of ion in one
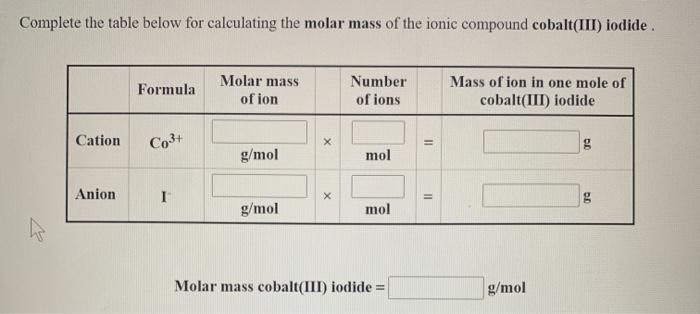
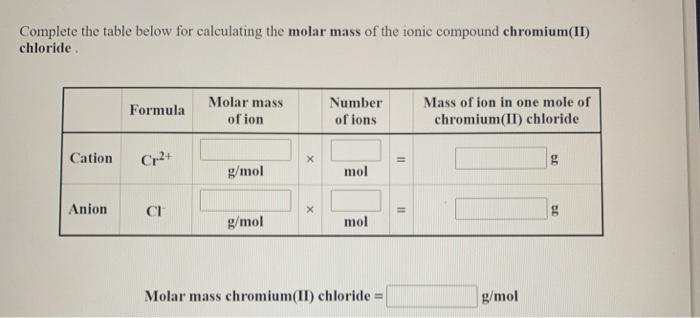
Complete the table below for calculating the molar mass of the ionic compound cobalt(III) iodide. Molar mass of ion Mass of ion in one mole of Formula Number of ions cobalt(III) iodide Cation Co3+ g g/mol mol Anion I g g/mol mol Molar mass cobalt(III) iodide = 2 X 11 II g/mol Complete the table below for calculating the molar mass of the ionic compound chromium(II) chloride. Formula Molar mass of ion Number of ions Mass of ion in one mole of chromium(II) chloride Cation Cr2+ X g g/mol mol Anion CH X g g/mol mol Molar mass chromium(II) chloride = 11 11 g/mol
Step by Step Solution
3.52 Rating (152 Votes )
There are 3 Steps involved in it
Solution 1 Molar mass of Tonic Compound Cobal klin ... View full answer

Get step-by-step solutions from verified subject matter experts


