Question: Complete the table below for zero, first and simple second order reactions. k, 1/[A]o 1/[A] vs. t -k, In [A]o [A]: vs. t -k,
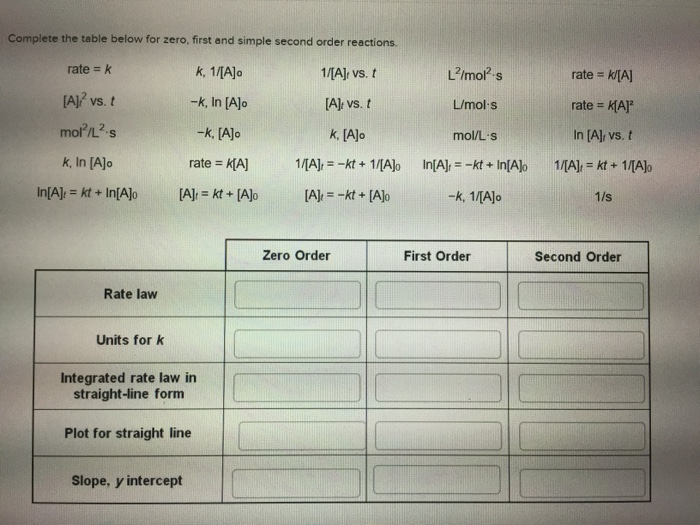
Complete the table below for zero, first and simple second order reactions. k, 1/[A]o 1/[A] vs. t -k, In [A]o [A]: vs. t -k, [A]o k, [A]o rate = K[A] [A] = kt+ [A]o rate = k [A]2 vs. t mol/L-s k, In [A]o In[A] = kt + In[A]o Rate law Units for k Integrated rate law in straight-line form Plot for straight line Slope, y intercept 1/[A] -kt + 1/[A]o [A] = -kt + [A]o Zero Order L/mol-s L/mol s mol/L-s In[A] = -kt + In[A]o -k, 1/[A]o First Order rate= k/[A] rate = K[A] In [A], vs. t 1/[A] = kt + 1/[A]o 1/s Second Order
Step by Step Solution
3.52 Rating (145 Votes )
There are 3 Steps involved in it
Rate Law Units for k Entegrate rate low in st l... View full answer

Get step-by-step solutions from verified subject matter experts


