Question: Complete the table below indicating the shift in equilibrium position and explanation for your choice for the following reaction: C2H4(g)+H2(g)C2H6(g)H=137.1k//mol
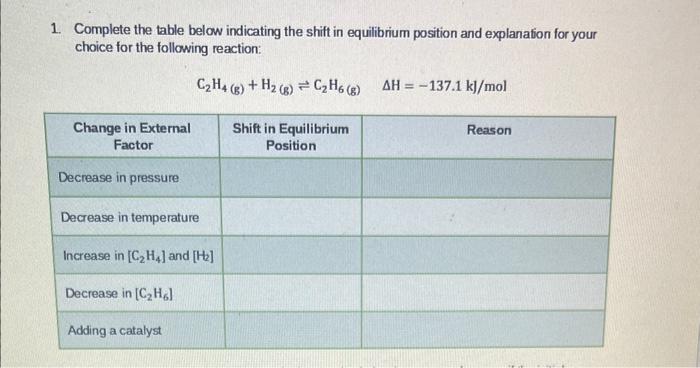
Complete the table below indicating the shift in equilibrium position and explanation for your choice for the following reaction: C2H4(g)+H2(g)C2H6(g)H=137.1k//mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


