Question: Complete the table below with the correct molecularity and rate law for each elementary reaction. Unimolecular rate= K[AP Elementary Step A products 2A ->
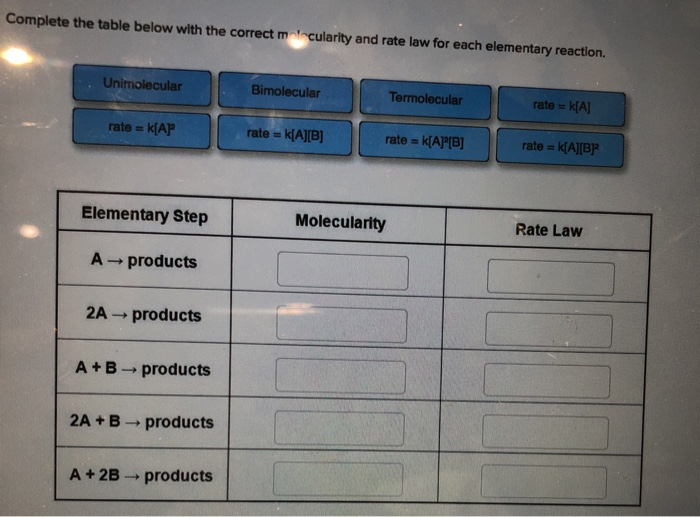
Complete the table below with the correct molecularity and rate law for each elementary reaction. Unimolecular rate= K[AP Elementary Step A products 2A -> products A+B products 2A + B products -> A + 2B - products Bimolecular rate == k[A][B] Termolecular rate == Molecularity IN k[A][B] rate = k[A] rate == k[A][B] Rate Law
Step by Step Solution
3.49 Rating (152 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


