Question: Complete the Table with the Following Data: Equation (1) Ba(HPO4)2: XBa Equation (2) BaHPO4: = = Equation (3) Ba3(PO4)2: XBa = ,2+ 1 mol Ba
Complete the Table with the Following Data:
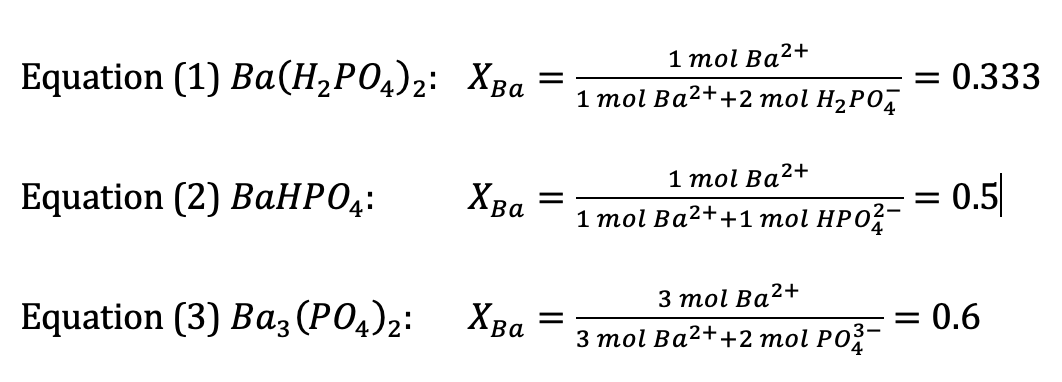
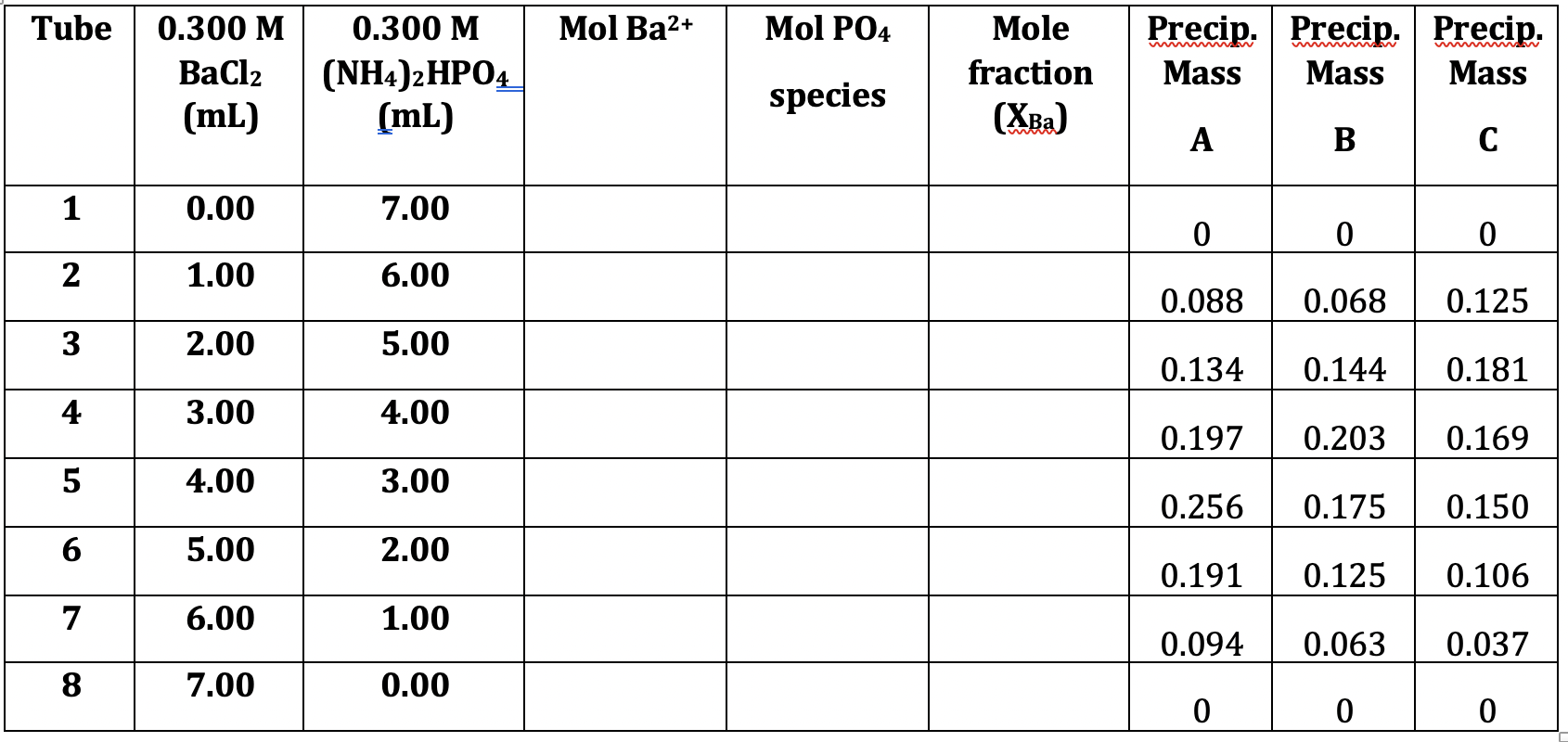
Equation (1) Ba(HPO4)2: XBa Equation (2) BaHPO4: = = Equation (3) Ba3(PO4)2: XBa = ,2+ 1 mol Ba 1 mol Ba+ + 2 mol HPO4 1 mol Ba+ 1 mol Ba+ +1 mol HP02- 2+ 3 mol Ba 3 mol Ba+ + 2 mol PO3- = 0.333 = 0.5 0.6
Step by Step Solution
★★★★★
3.43 Rating (153 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

formula no of moles MXV 1000 example Case2 no of moles POCO SHOT ON POCO M2 4 3 5 Mole fract... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


