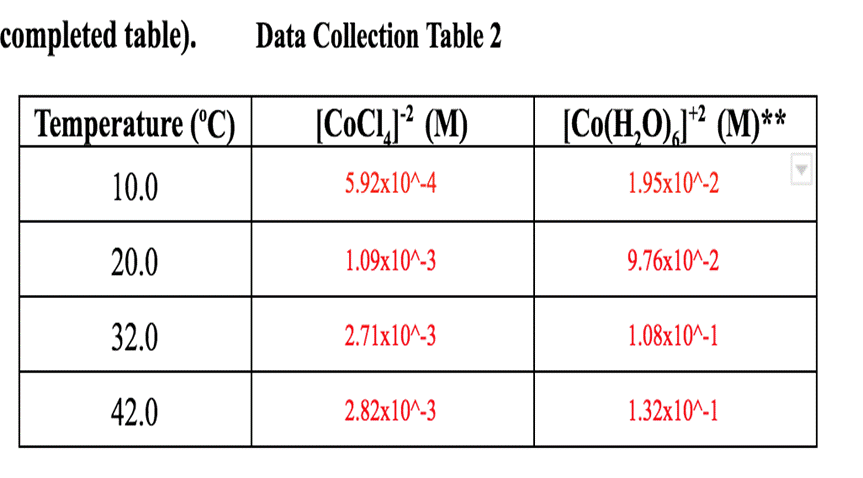
Question: completed table). Data Collection Table 2 [CoCl (M) 5.92x10^-4 Temperature (C) 10.0 20.0 32.0 42.0 1.09x10^-3 2.71x10^-3 2.82x10^-3 [Co(HO),]* (M)** 1.95x10^-2 9.76x10^-2 1.08x10^-1 1.32x10^-1

![(4 points for completed table). Data Collection Table 3 10.0 20.0 32.0 42.0 Temp. [CoC14]-2 [Co(H;0)]+2 [CI]-](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2022/03/6239a5151ae5c_9806239a514aafe5.jpg)
completed table). Data Collection Table 2 [CoCl (M) 5.92x10^-4 Temperature (C) 10.0 20.0 32.0 42.0 1.09x10^-3 2.71x10^-3 2.82x10^-3 [Co(HO),]* (M)** 1.95x10^-2 9.76x10^-2 1.08x10^-1 1.32x10^-1 2. Complete table 3 below. Use an ICE table to calculate the equilibrium concentrations of [Co(HO), and Cr. Assume the initial concentration of [Co(HO), is 0.0500 M and the initial concentration of Cl is 0.100 M . Use the equilibrium concentration of [CoC1,] from Table 2. Then use your equilibrium concentrations to calculate the equilibrium constant at all temperatures. (4 points for completed table). Data Collection Table 3 10.0 20.0 32.0 42.0 Temp. [CoC14]-2 [Co(H;0)]+2 [CI]- Keq
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
To complete Table 3 we need to calculate the equilibrium concentratio... View full answer

Get step-by-step solutions from verified subject matter experts


