Question: Condition to be satisfied for a and phases to be in equilibrium in a two- component (A and B) system at constant temperature and
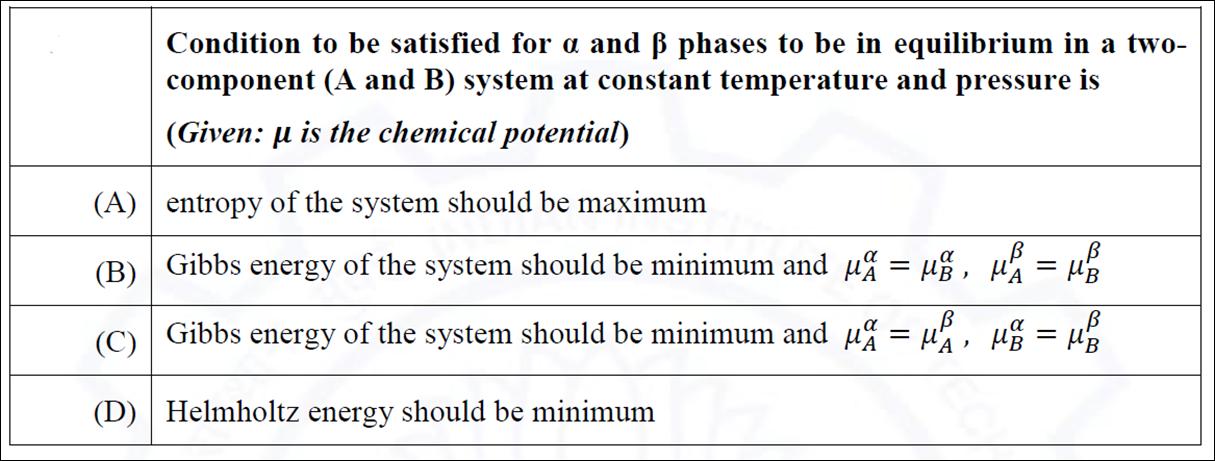
Condition to be satisfied for a and phases to be in equilibrium in a two- component (A and B) system at constant temperature and pressure is (Given: is the chemical potential) entropy of the system should be maximum Gibbs energy Gibbs energy of the system should be minimum and = , g = B (A) (B) (C) (D) Helmholtz energy should be minimum B of the system should be minimum and = , =
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
The detailed answer for the above ques... View full answer

Get step-by-step solutions from verified subject matter experts


