Question: Conducts electricity in solid phase only Has a low melting point Conducts electricity in the aqueous phase only Soluble in Non Polar Solvent Exhibits magnetism
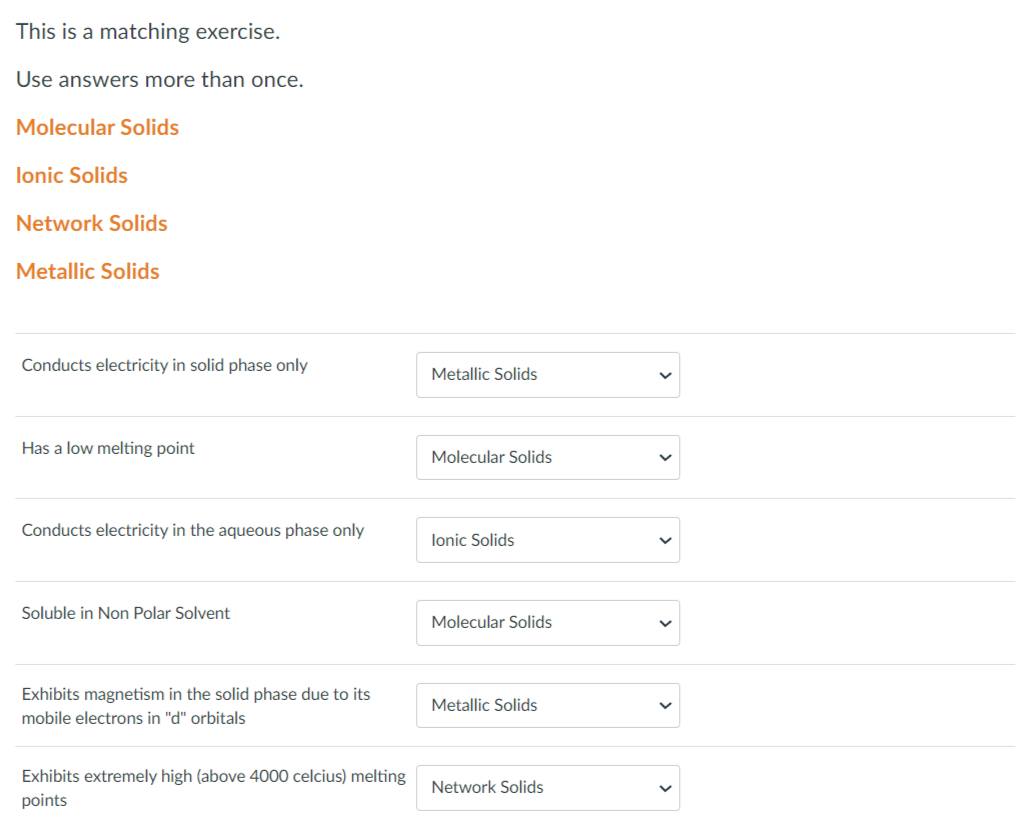
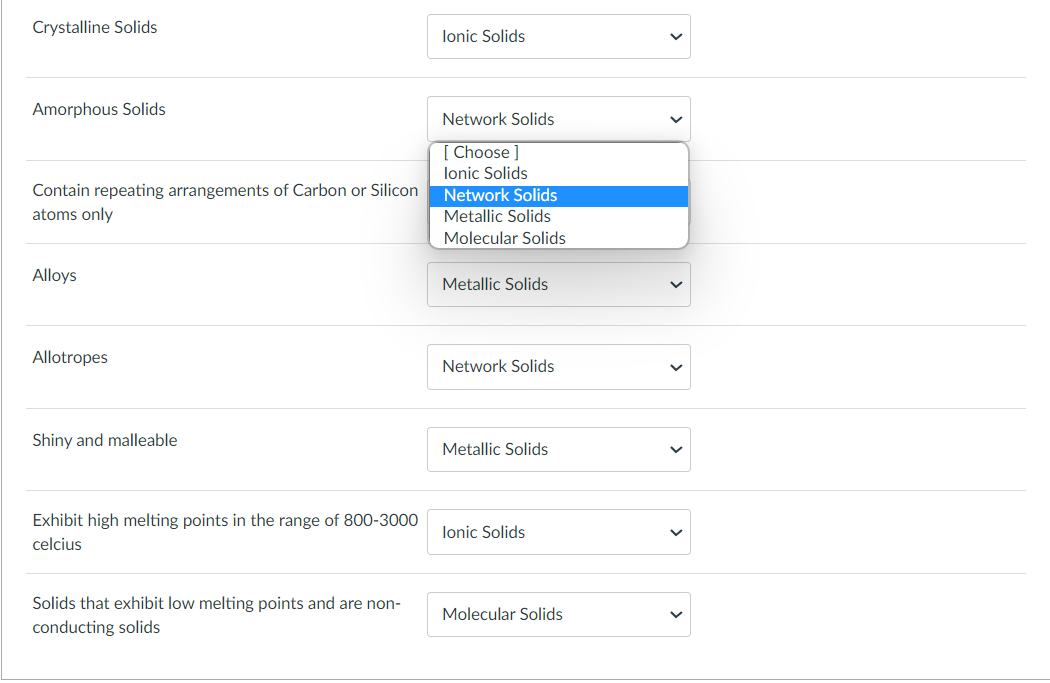
Conducts electricity in solid phase only Has a low melting point Conducts electricity in the aqueous phase only Soluble in Non Polar Solvent Exhibits magnetism in the solid phase due to its mobile electrons in "d" orbitals Exhibits extremely high (above 4000 celcius) melting points Amorphous Solids Contain repeating arrangements of Carbon or Silicon atoms only Alloys Allotropes Shiny and malleable Exhibit high melting points in the range of 8003000 celcius Solids that exhibit low melting points and are nonconducting solids
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


