Question: confused on what question 2 is asking 2. Using the following major ion composition of world-averaged river water and the Davies formula for calculating ion
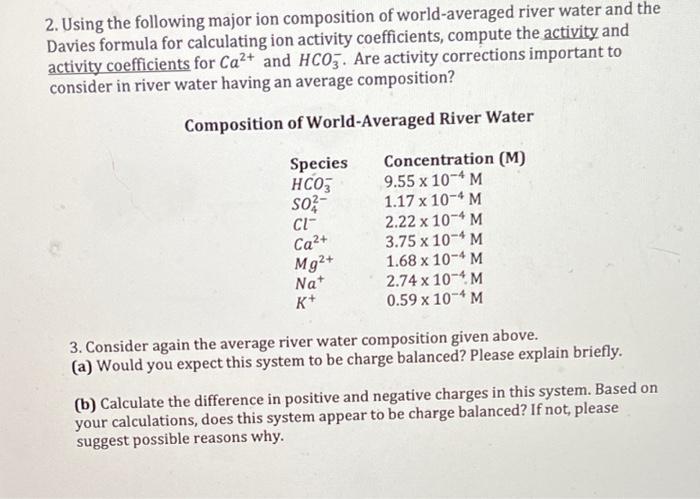
2. Using the following major ion composition of world-averaged river water and the Davies formula for calculating ion activity coefficients, compute the activity and activity coefficients for Ca2+ and HCO3. Are activity corrections important to consider in river water having an average composition? Composition of World-Averaged River Water Species ; S02 Cl" Ca2+ Mg2+ Nat K+ Concentration (M) 9.55 x 10-4 M 1.17 x 10-4 M 2.22 x 10-4 M 3.75 x 10-4 M 1.68 x 10-4 M 2.74 x 10-4 M 0.59 x 10-6 M 3. Consider again the average river water composition given above. (a) Would you expect this system to be charge balanced? Please explain briefly. (b) Calculate the difference in positive and negative charges in this system. Based on your calculations, does this system appear to be charge balanced? If not, please suggest possible reasons why
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


