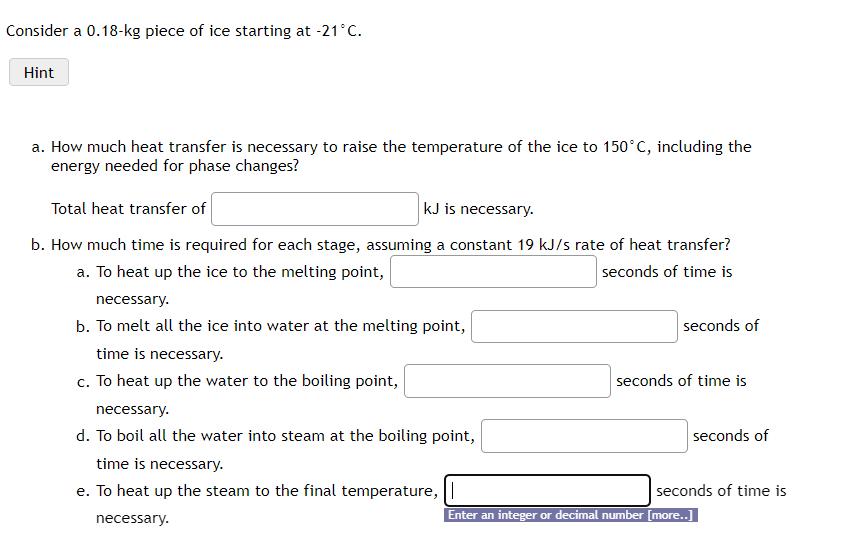
Question: Consider a 0 . 1 8 - k g piece of ice starting at - 2 1 C . a . How much heat transfer
Consider a piece of ice starting at
a How much heat transfer is necessary to raise the temperature of the ice to including the energy needed for phase changes?
Total heat transfer of is necessary.
b How much time is required for each stage, assuming a constant rate of heat transfer?
a To heat up the ice to the melting point, seconds of time is necessary.
b To melt all the ice into water at the melting point, seconds of time is necessary.
c To heat up the water to the boiling point, seconds of time is necessary.
d To boil all the water into steam at the boiling point, seconds of time is necessary.
e To heat up the steam to the final temperature, seconds of time is necessary.
Enter an integer or decimal number more
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


