Question: Consider a box with two gases separated by an impermeable membrane. The membrane can move back and forth, but the gases cannot penetrate the

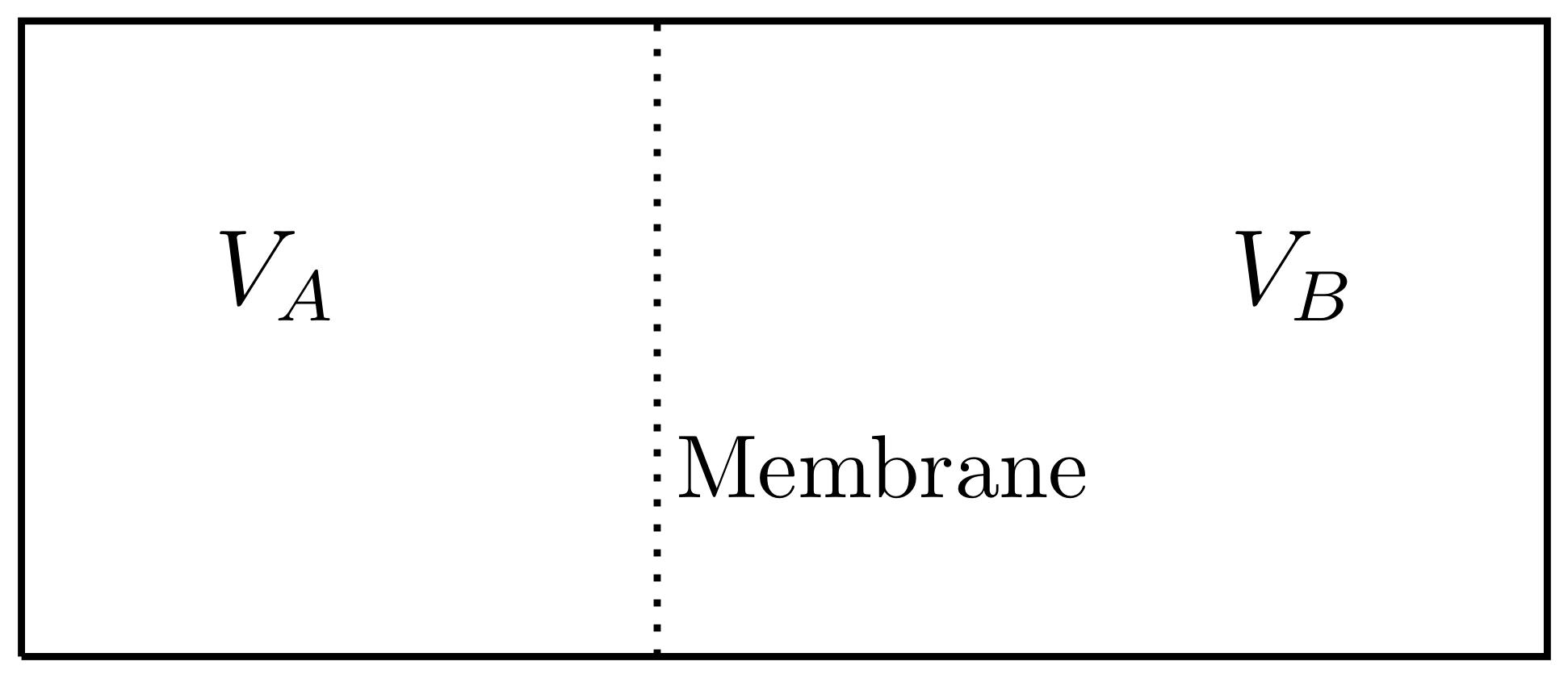
Consider a box with two gases separated by an impermeable membrane. The membrane can move back and forth, but the gases cannot penetrate the membrane. The left side is filled with gas A and the right side is filled with gas B. We will assume that equipartition applies to both gases, but gas A has an excluded volume due to large molecules so its entropy has a different formula. SA=NAkln(VA?bNA)+f(UA,NA) SB=NBkln(VB)+f(UB,NB) Keep in mind that the total box volume is constant, so VA+VB=constantVA+VB=constant. 1) If NA= 1 moles, NB = 2 moles, the total volume of the box is 1 m3, and b= 4 x 10-4 m3/mole, then find the equilibrium value of VA by maximizing the total entropy. VA VB Membrane
Step by Step Solution
3.40 Rating (153 Votes )
There are 3 Steps involved in it
from ideal gas law PA PB PA VA NAb ... View full answer

Get step-by-step solutions from verified subject matter experts


