Question: Consider a cell: Sn(s) + Cu2+(aq) Sn2+(aq) + Cu(s) in which the concentration of [Cu2+] and [Sn2+] are both 2M. Calculate the theoretical cell potential.
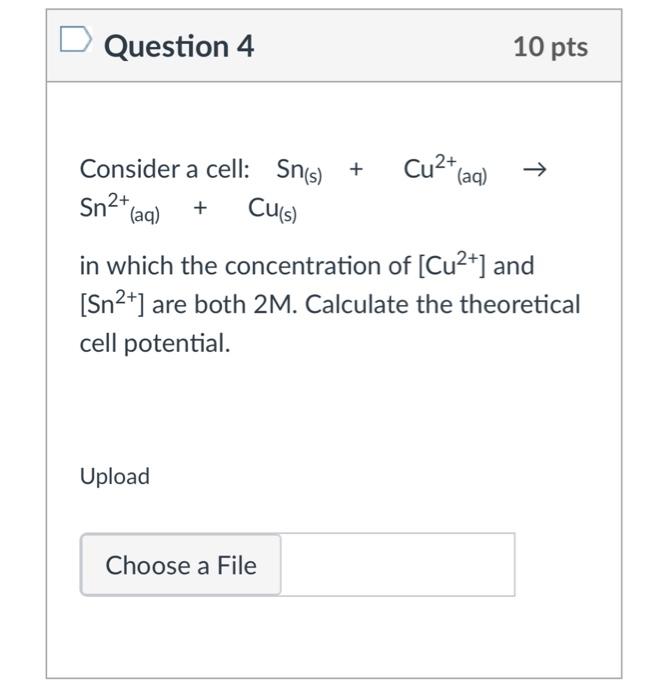
Question 4 10 pts Cu2+ (aq) Consider a cell: Sn(s) + + Sn2+ (aq) Cu(s) in which the concentration of [Cu2+] and [Sn2+] are both 2M. Calculate the theoretical cell potential. Upload Choose a File
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


