Question: Consider a container with a frictionless piston that contains a given amount of an ideal gas. If the external pressure is kept constant, the piston
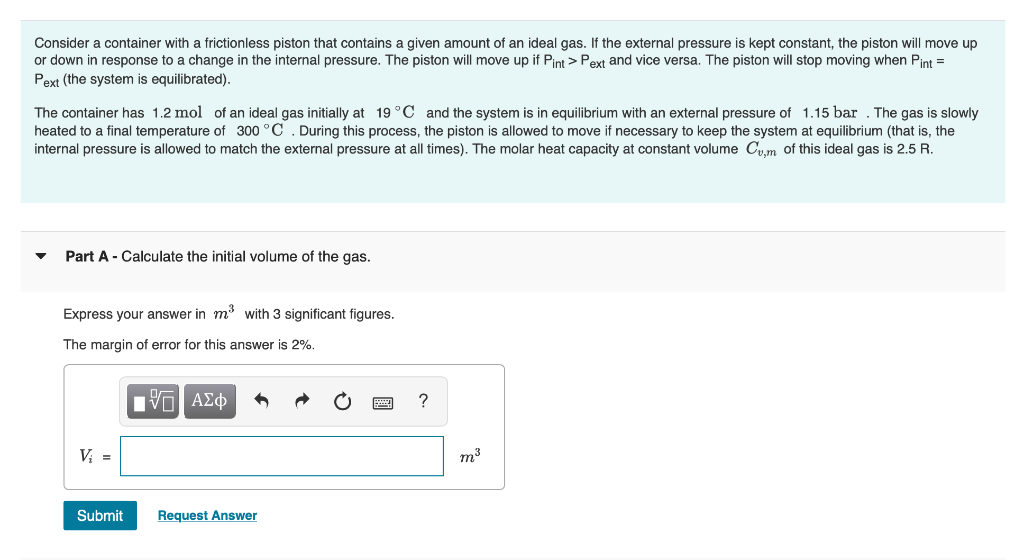
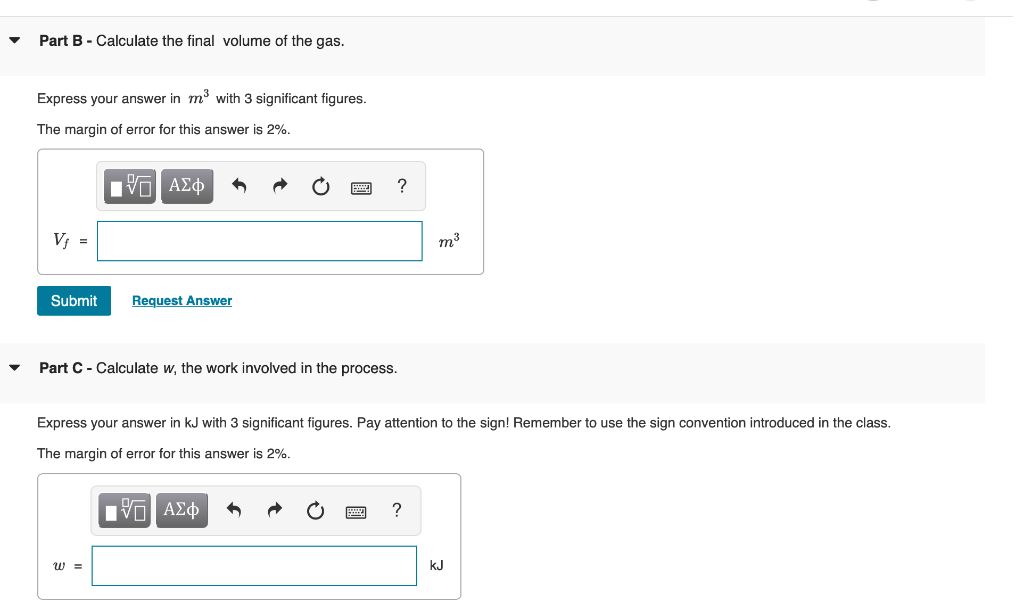
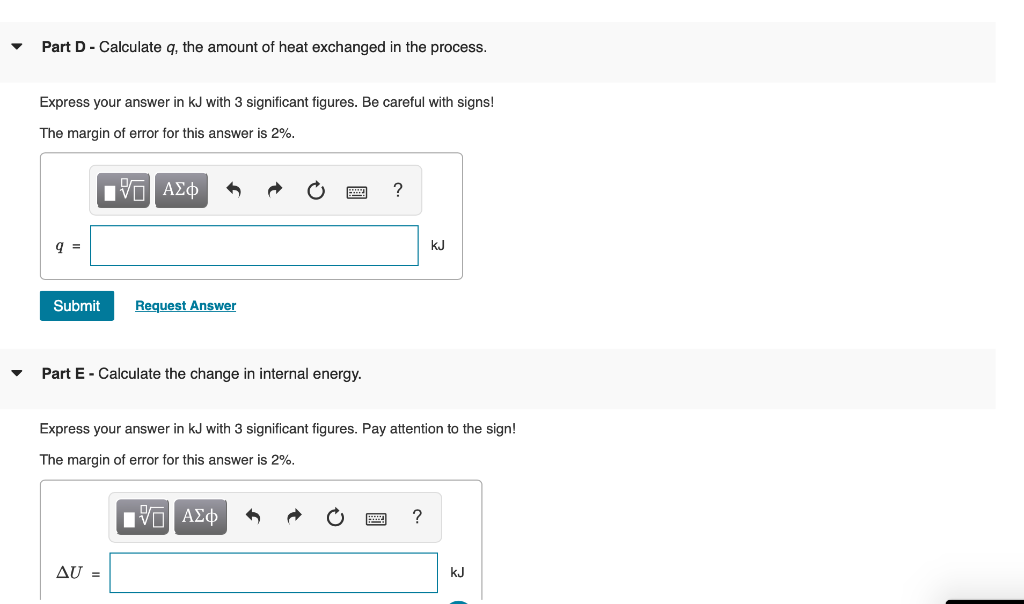
Consider a container with a frictionless piston that contains a given amount of an ideal gas. If the external pressure is kept constant, the piston will move up or down in response to a change in the internal pressure. The piston will move up if Pint > Pext and vice versa. The piston will stop moving when Pint = Pext (the system is equilibrated). The container has 1.2 mol of an ideal gas initially at 19C and system is in equilibrium with an external pressure of 1.15 bar . The gas is slowly heated to a final temperature of 300 C . During this process, the piston is allowed to move if necessary to keep the system at equilibrium (that is, the internal pressure is allowed to match the external pressure at all times). The molar heat capacity at constant volume Cum of this ideal gas is 2.5 R. Part A - Calculate the initial volume of the gas. Express your answer in ms with 3 significant figures. The margin of error for this answer is 2%. ? Vi = m3 Submit Request Answer 7 Part B - Calculate the final volume of the gas. Express your answer in my with 3 significant figures. The margin of error for this answer is 2%. 0 EVO AEO ? Vi = m3 Submit Request Answer Part C - Calculate w, the work involved in the process. Express your answer in kJ with 3 significant figures. Pay attention to the sign! Remember to use the sign convention introduced in the class. The margin of error for this answer is 2%. EVO AED ? W = kJ Part D - Calculate q, the amount of heat exchanged in the process. Express your answer in kJ with 3 significant figures. Be careful with signs! The margin of error for this answer is 2%. IVO AL ? 9 = kJ Submit Request Answer Part E - Calculate the change in internal energy. Express your answer in kJ with 3 significant figures. Pay attention to the sign! The margin of error for this answer is 2%. ? AU = kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


