Question: Consider a d'ion, V+ that appears in numerous complexes as VO+. The level scheme is given in the figure and the singly occupied orbital (ground
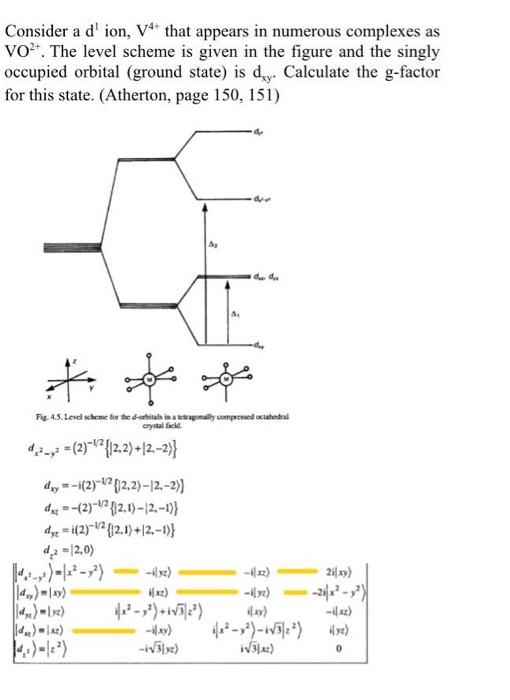
Consider a d'ion, V+ that appears in numerous complexes as VO+. The level scheme is given in the figure and the singly occupied orbital (ground state) is dry. Calculate the g-factor for this state. (Atherton, page 150, 151) dy d. * so Fig. 4.5. Lovel scheme for the dubitats in a tetragonally compreso octahedral crystal 42--(2)***{{2.2)+12.-2}} d. =-i(2)-V?[12,2)-12.-2) des --(2)=\{12.1)-12-19 dyz = i(2)-Y{12,1)+12.-1) 4,2 =2,0) 21|xy) - 1x3y) 131.4) 0 Consider a d'ion, V+ that appears in numerous complexes as VO+. The level scheme is given in the figure and the singly occupied orbital (ground state) is dry. Calculate the g-factor for this state. (Atherton, page 150, 151) dy d. * so Fig. 4.5. Lovel scheme for the dubitats in a tetragonally compreso octahedral crystal 42--(2)***{{2.2)+12.-2}} d. =-i(2)-V?[12,2)-12.-2) des --(2)=\{12.1)-12-19 dyz = i(2)-Y{12,1)+12.-1) 4,2 =2,0) 21|xy) - 1x3y) 131.4) 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


