Question: Consider a gas in a piston-cylinder device going through a cyclic process. The work output from this piston-cylinder device per cycle is 19kJ. The cycle
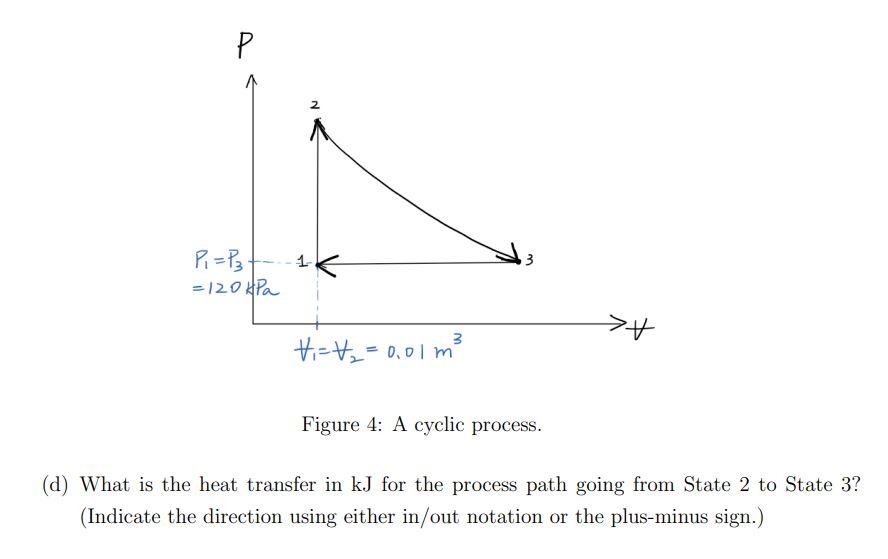
Consider a gas in a piston-cylinder device going through a cyclic process. The work output from this piston-cylinder device per cycle is 19kJ. The cycle contains three process paths: - State 1 to State 2: isochoric process (V1=V2=0.01m3) - State 2 to State 3: U2=U3, where U is the internal energy of the gas - State 3 to State 1: isobaric process (P1=P3=120kPa) with the amount of work input to the system of 6kJ (a) Which process path does not involve work interaction? Explain. (b) What is the volume of the gas (V3) in m3 at State 3 ? (c) What is the work interaction in kJ for the process path going from State 2 to State 3 ? (Indicate the direction using either in/out notation or the plus-minus sign.) Figure 4: A cyclic process. (d) What is the heat transfer in kJ for the process path going from State 2 to State 3 ? (Indicate the direction using either in/out notation or the plus-minus sign.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


