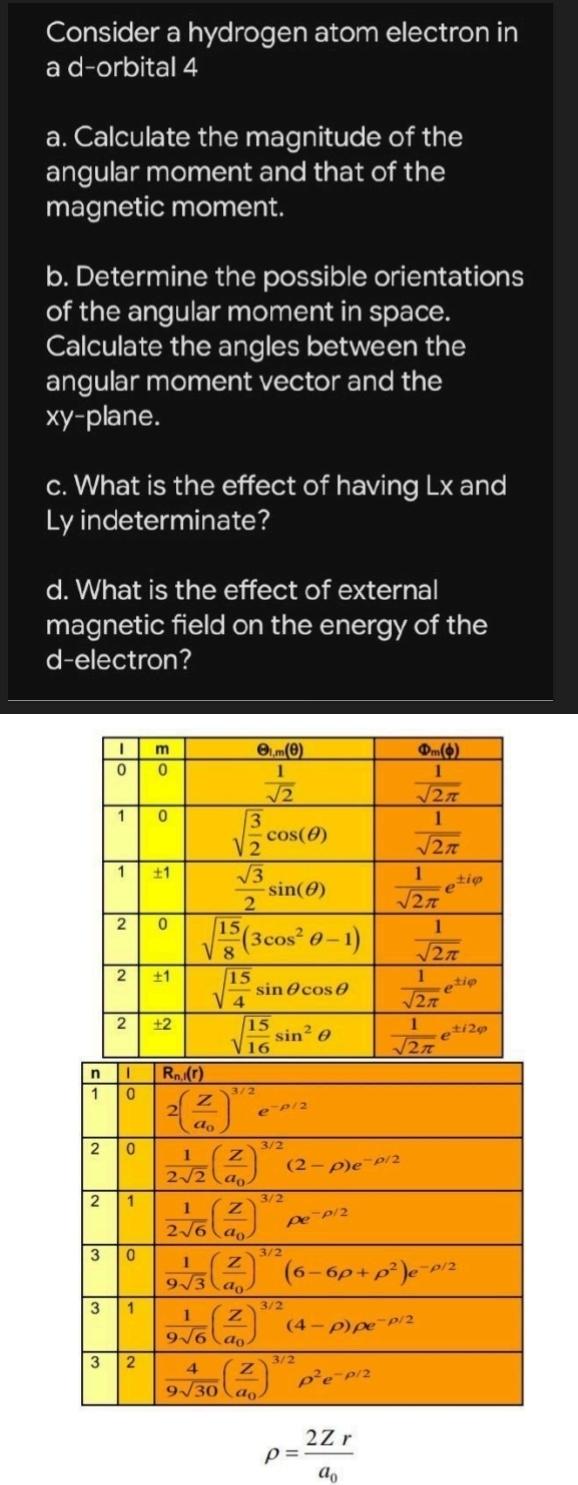
Question: Consider a hydrogen atom electron in a d - orbital a . Calculate the magnitude of the angular moment and that of the magnetic moment.
Consider a hydrogen atom electron in a dorbital
a Calculate the magnitude of the angular moment and that of the magnetic moment.
b Determine the possible orientations of the angular moment in space. Calculate the angles between the angular moment vector and the plane.
c What is the effect of having and Ly indeterminate?
d What is the effect of external magnetic field on the energy of the delectron?
table
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


