Question: Consider a Li-S-O system. The Gibbs triangle and electrical potentials of each sub-triangle are given below. Atomic weights of Li, S and O are 6.94,
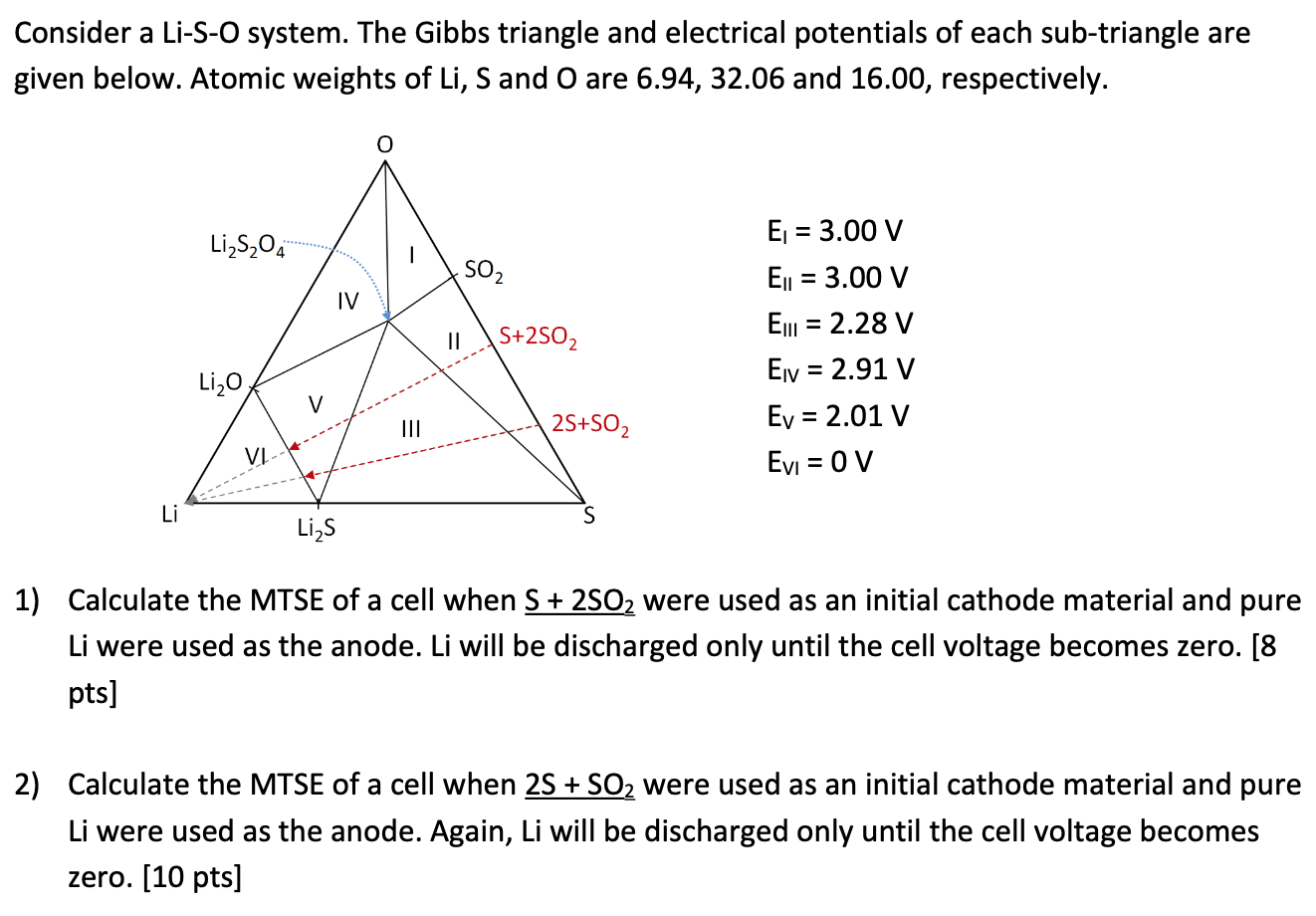
Consider a Li-S-O system. The Gibbs triangle and electrical potentials of each sub-triangle are given below. Atomic weights of Li, S and O are 6.94, 32.06 and 16.00, respectively. Li2S204 SO2 IV - I/ S+2502 Ej = 3.00 V Ej = 3.00 V El = 2.28 V Eiv = 2.91 V Ev = 2.01 V Evi = 0 V Li2O V 25+S02 = Li S Li2S 1) Calculate the MTSE of a cell when S + 2502 were used as an initial cathode material and pure Li were used as the anode. Li will be discharged only until the cell voltage becomes zero. [8 pts] 2) Calculate the MTSE of a cell when 2S + SO2 were used as an initial cathode material and pure Li were used as the anode. Again, Li will be discharged only until the cell voltage becomes zero. [10 pts]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


