Question: Consider a reaction with reactant A, giving products: A> products Suppose the rate of reaction is 0.04M/sec when [A]=0.23M.The concentration of A is doubled to
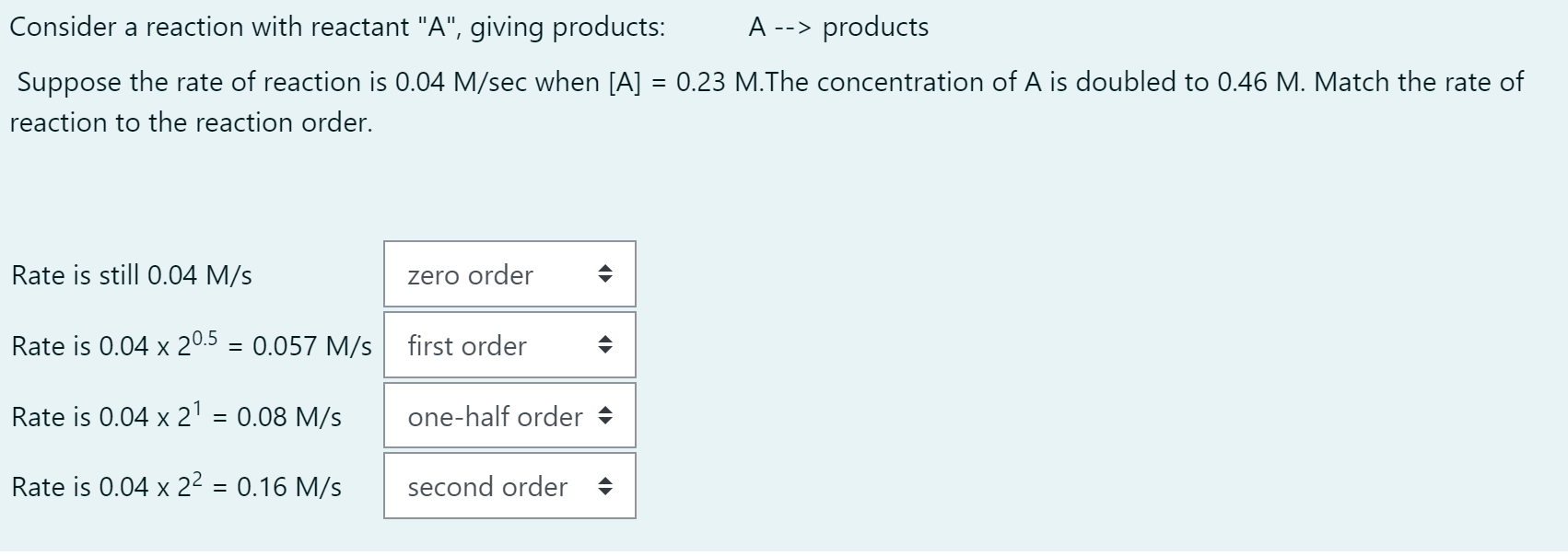
Consider a reaction with reactant "A", giving products: A> products Suppose the rate of reaction is 0.04M/sec when [A]=0.23M.The concentration of A is doubled to 0.46M. Match the rate of reaction to the reaction order. Rate is still 0.04M/s Rate is 0.0420.5=0.057M/s Rate is 0.0421=0.08M/s Rate is 0.0422=0.16M/s
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


