Question: Consider a reversible elementary gas phase reaction. A 2. reaction has the standard Gibbs free energy of the reaction of 50caW mol and standard enthalpy
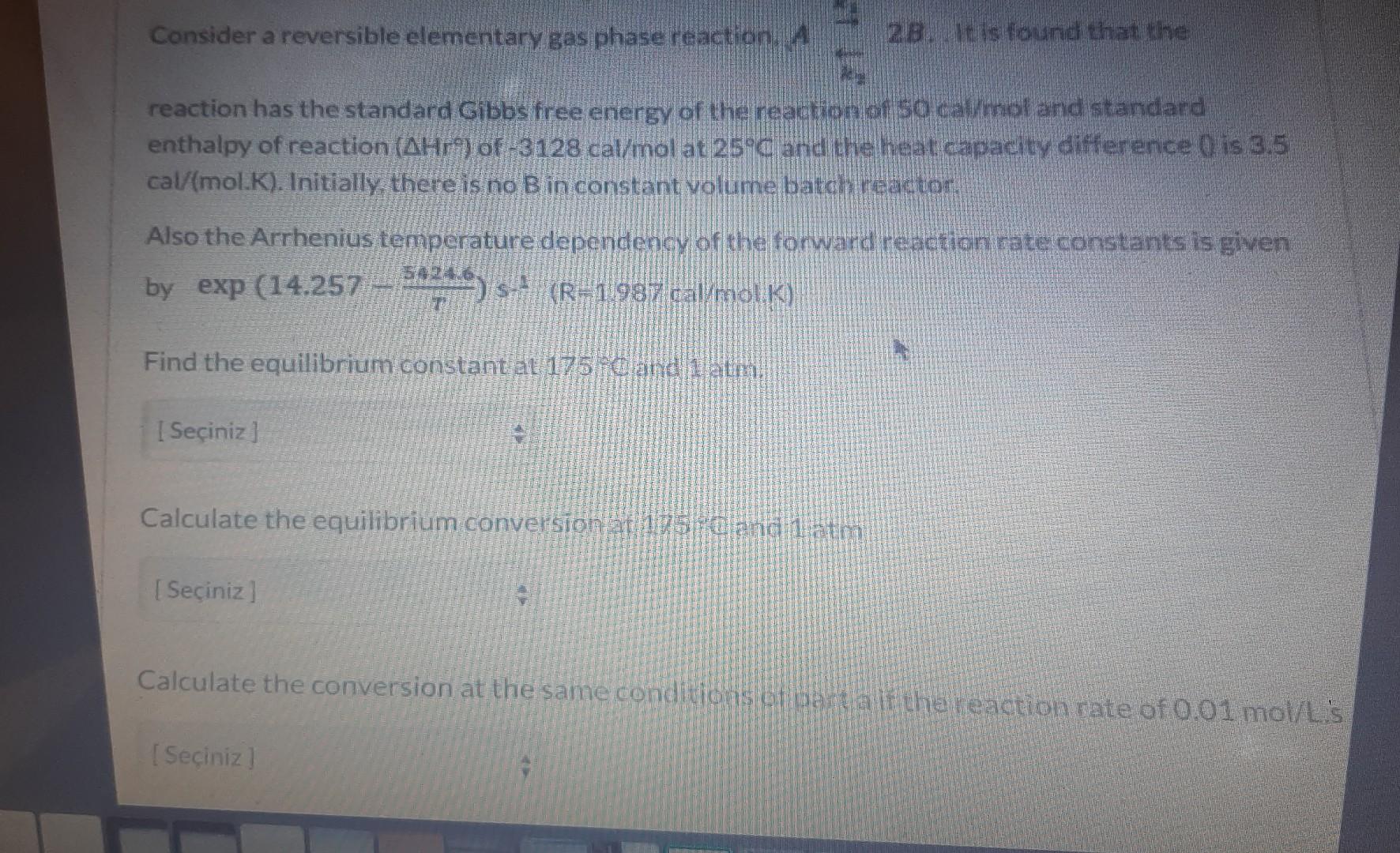
Consider a reversible elementary gas phase reaction. A 2. reaction has the standard Gibbs free energy of the reaction of 50caW mol and standard enthalpy of reaction (Hr) of 3128cal/mol at 25C and the heat capacity difference 0 is 3.5 cal( mol.K). Initially there is no B in constant volume batch reactor: Also the Arrhenius temperature dependency of the forward reaction rate constants is given by exp(14.257T54246)s(R1987cal(molK) Find the equilibrium constantat 175 ic and 1 atm. Calculate the equilibrium conversionat 105 ioc and 1 atm Calculate the conversion at the same condivions of partaif the reaction rate of 0.01mol L.'s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


