Question: Consider a simple cubic (a.k.a, primitive cubic) unit cell as shown here. How many atoms are shown in this image? What fraction of each
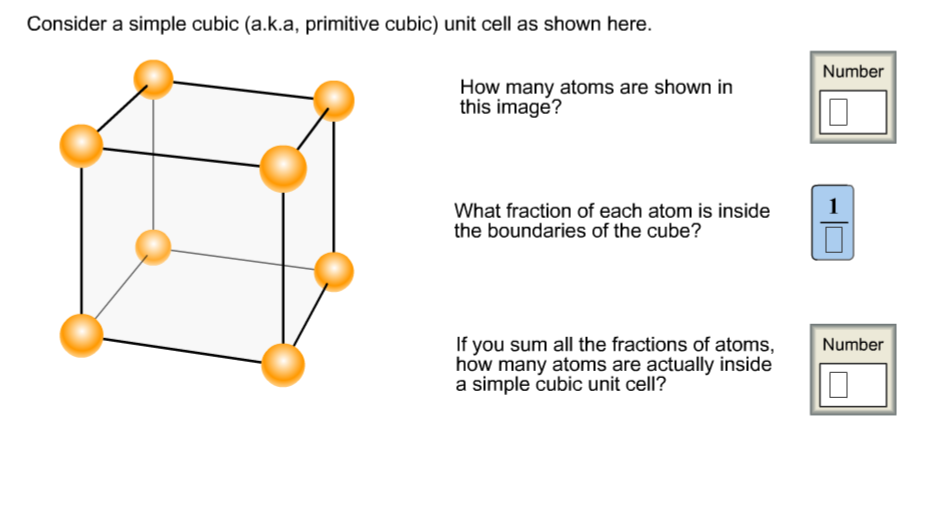
Consider a simple cubic (a.k.a, primitive cubic) unit cell as shown here. How many atoms are shown in this image? What fraction of each atom is inside the boundaries of the cube? If you sum all the fractions of atoms, how many atoms are actually inside a simple cubic unit cell? Number 1 Number
Step by Step Solution
3.53 Rating (146 Votes )
There are 3 Steps involved in it
The given unit cell is a simple cubic unit cell No o... View full answer

Get step-by-step solutions from verified subject matter experts


