Question: Consider a thermodynamic function J defined as JS-T (1) (a) :Using differentials and the laws of thermodynamics, determine the follow- ing quantities in terms of
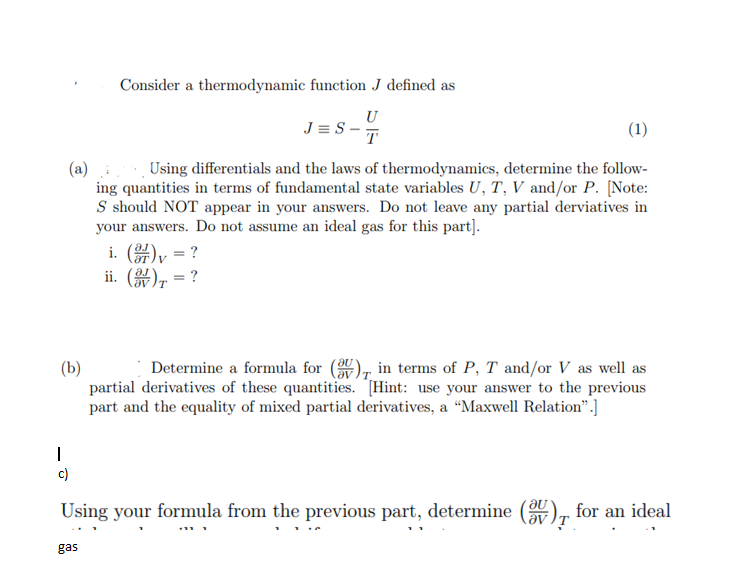
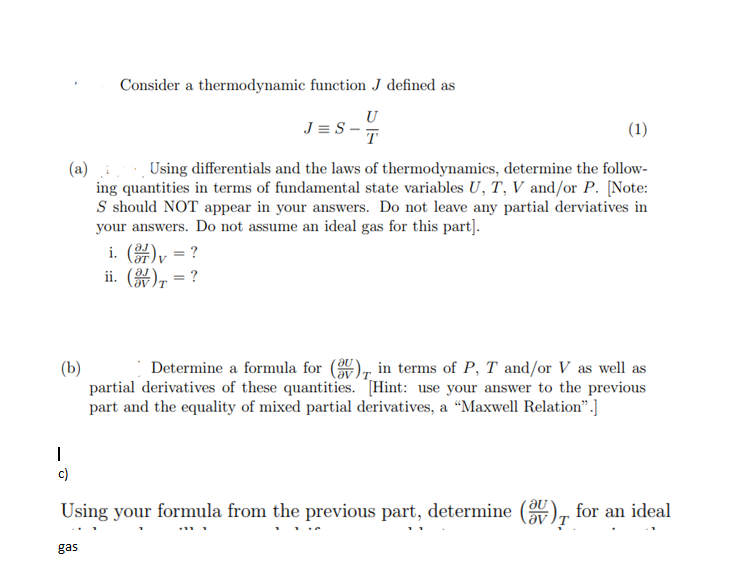
Consider a thermodynamic function J defined as JS-T (1) (a) :Using differentials and the laws of thermodynamics, determine the follow- ing quantities in terms of fundamental state variables U, T, V and/or P. [Note: S should NOT appear in your answers. Do not leave any partial derviatives in your answers. Do not assume an ideal gas for this part]. i. (arly = ? ii. (84) = ? (b) Determine a formula for (ay ), in terms of P, T and/or V as well as partial derivatives of these quantities. Hint: use your answer to the previous part and the equality of mixed partial derivatives, a "Maxwell Relation".] 2 - Using your formula from the previous part, determine (av ), for an ideal . . gas
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


