Question: Consider an electron in the hydrogen atom. The electron is initially in the ground state. Assume that a certain process puts the electron in a
Consider an electron in the hydrogen atom. The electron is initially in the ground state.
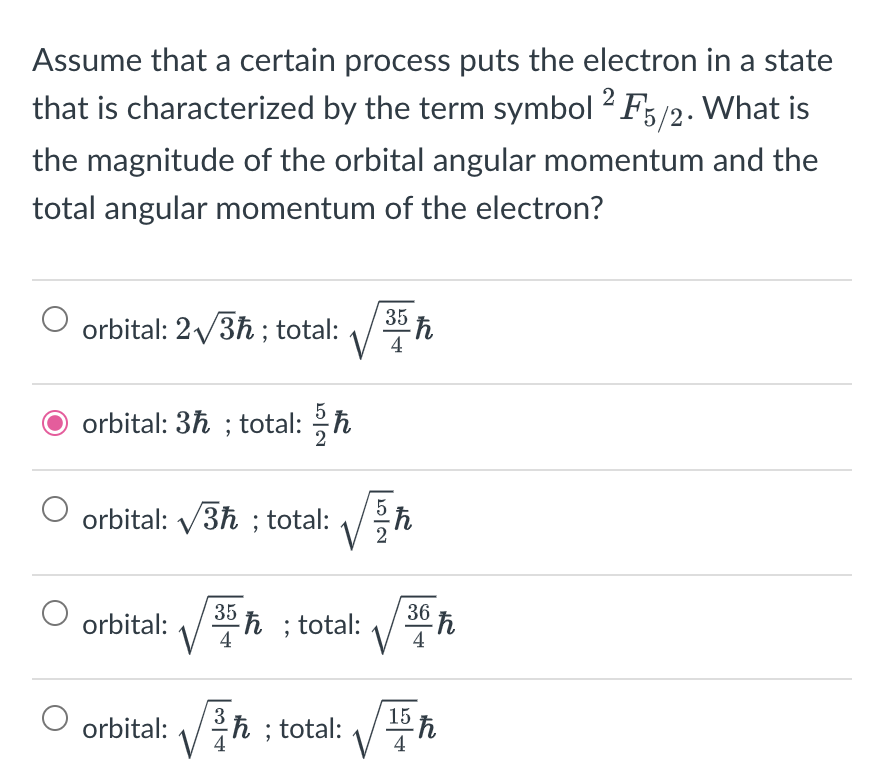
Assume that a certain process puts the electron in a state that is characterized by the term symbol 2F5/2. What is the magnitude of the orbital angular momentum and the total angular momentum of the electron? orbital: 23; total: 435 orbital: 3; total: 25 orbital: 3; total: 25 orbital: 435; total: 436 orbital: 43; total: 415
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


