Question: Consider an electron that can exist only between ( x = 0 ) and ( x = L = 3 . 6
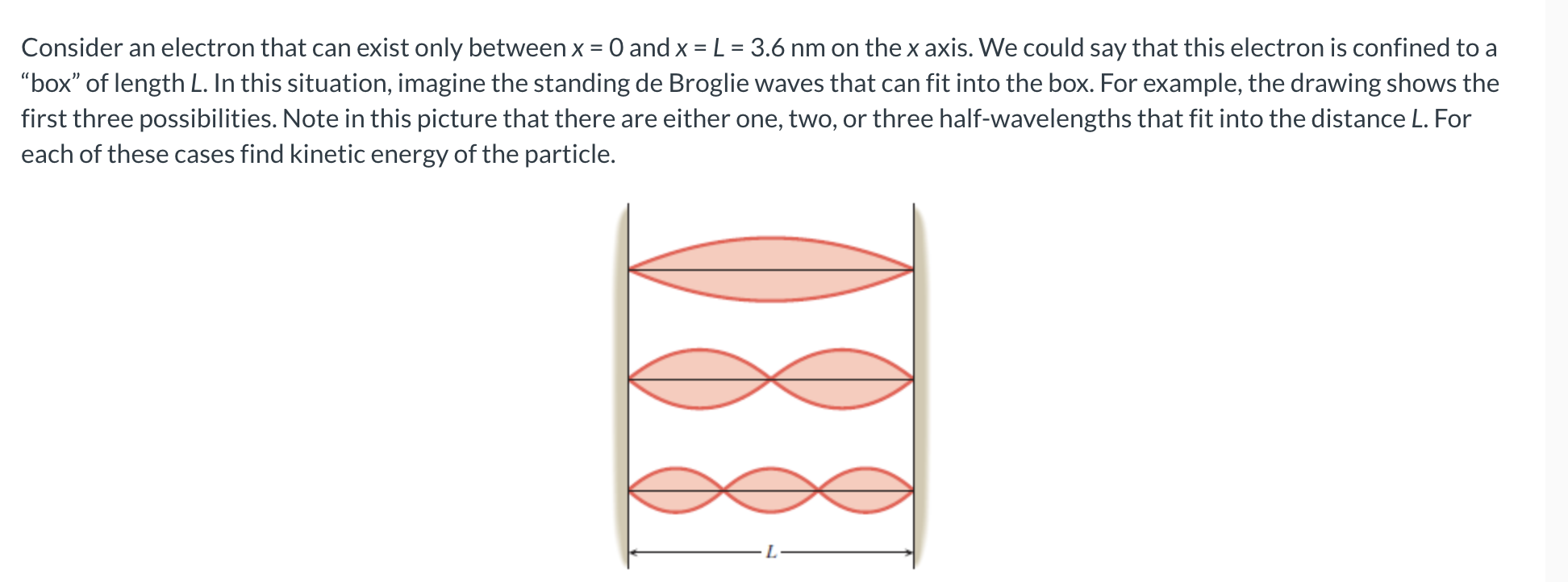
Consider an electron that can exist only between x and xLmathrm~nm on the x axis. We could say that this electron is confined to a "box" of length L In this situation, imagine the standing de Broglie waves that can fit into the box. For example, the drawing shows the first three possibilities. Note in this picture that there are either one, two, or three halfwavelengths that fit into the distance L For each of these cases find kinetic energy of the particle.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


