Question: Consider an ideal gas whose entropy is given by U 0+5 R ln +2R ln- n where n = number of moles, R =
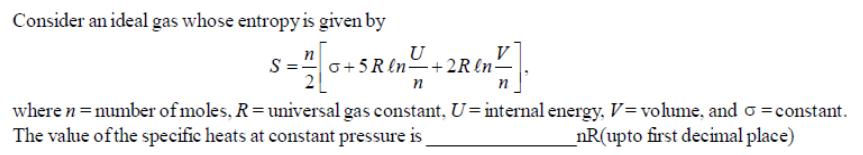
Consider an ideal gas whose entropy is given by U 0+5 R ln +2R ln- n where n = number of moles, R = universal gas constant, U-internal energy, V=volume, and constant. The value of the specific heats at constant pressure is _nR(upto first decimal place)
Step by Step Solution
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


