Question: Consider an isothermal CSTR with first order reaction with a rate constant k. Let V, v are the volume of liquid in the tank, and
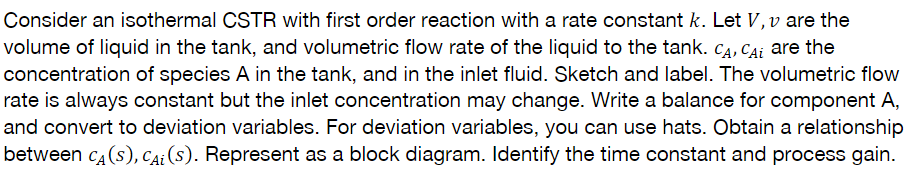
Consider an isothermal CSTR with first order reaction with a rate constant k. Let V, v are the volume of liquid in the tank, and volumetric flow rate of the liquid to the tank. Ca, Cai are the concentration of species A in the tank, and in the inlet fluid. Sketch and label. The volumetric flow rate is always constant but the inlet concentration may change. Write a balance for component A, and convert to deviation variables. For deviation variables, you can use hats. Obtain a relationship between c(s), cai(s). Represent as a block diagram. Identify the time constant and process gain. Consider an isothermal CSTR with first order reaction with a rate constant k. Let V, v are the volume of liquid in the tank, and volumetric flow rate of the liquid to the tank. Ca, Cai are the concentration of species A in the tank, and in the inlet fluid. Sketch and label. The volumetric flow rate is always constant but the inlet concentration may change. Write a balance for component A, and convert to deviation variables. For deviation variables, you can use hats. Obtain a relationship between c(s), cai(s). Represent as a block diagram. Identify the time constant and process gain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


