Question: Consider carbon is diffusing into -Fe. Compute the diffusion coefficient of carbon into a-Fe at 795C Given that Gas constant R= 8.314 J/mol-k Note: Round
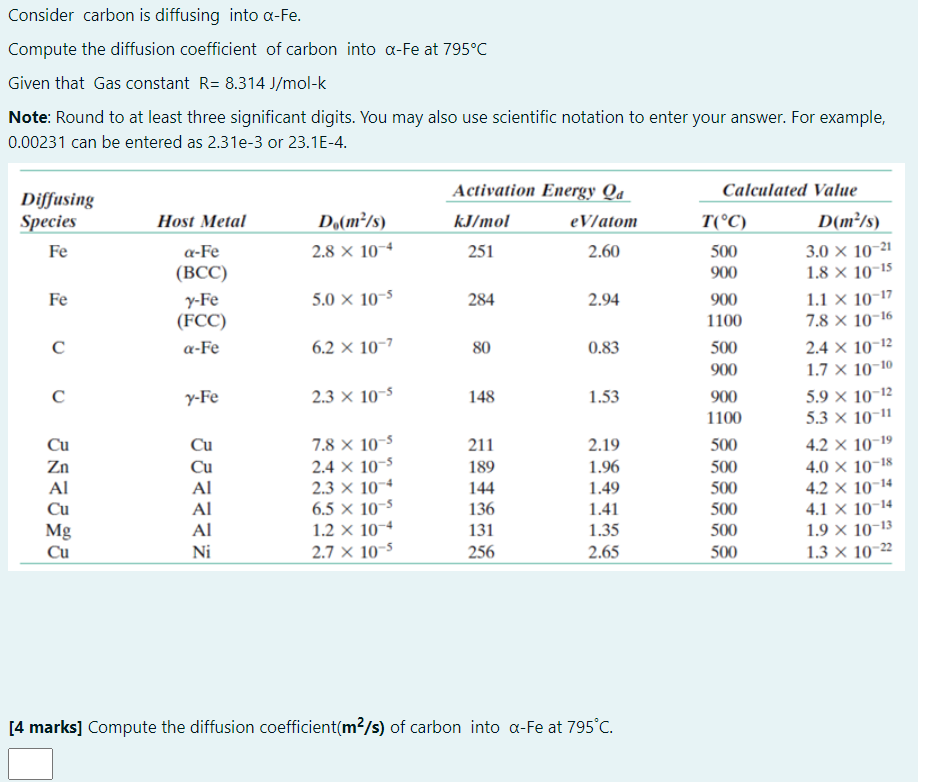
Consider carbon is diffusing into -Fe. Compute the diffusion coefficient of carbon into a-Fe at 795C Given that Gas constant R= 8.314 J/mol-k Note: Round to at least three significant digits. You may also use scientific notation to enter your answer. For example, 0.00231 can be entered as 2.31e-3 or 23.1E-4. Activation Energy Qa Calculated Value Diffusing Species Host Metal Do(m/s) kJ/mol eV/atom Fe a-Fe 2.8 x 10-4 251 2.60 (BCC) Fe y-Fe 5.0 x 10-5 284 2.94 (FCC) a-Fe 6.2 x 10-7 80 0.83 C y-Fe 2.3 x 10-5 148 1.53 Cu Cu 7.8 x 10-5 211 2.19 Cu 2.4 x 10-5 189 1.96 Al 2.3 10-4 144 1.49 6.5 x 10-5 136 1.41 Al 1.2 x 10-4 131 1.35 Ni 2.7 x 10-5 256 2.65 [4 marks] Compute the diffusion coefficient (m/s) of carbon into -Fe at 795C. Zn Al Cu Mg Cu T(C) 500 900 900 1100 500 900 900 1100 500 500 500 500 500 500 D(m/s) 3.0 x 10-21 1.8 X 10-15 1.1 X 10-17 7.8 X 10-16 2.4 x 10-12 1.7 X 10-10 5.9 x 10-12 5.3 10-11 4.2 x 10-19 4.0 10-18 4.2 x 10-14 4.1 X 10-14 1.9 X 10-13 1.3 x 10-22
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


