Question: Consider diffusion and reaction (A B) on a porous catalytic particle (slab of half- thickness L). The rate of reaction ra (of A disappearance) is
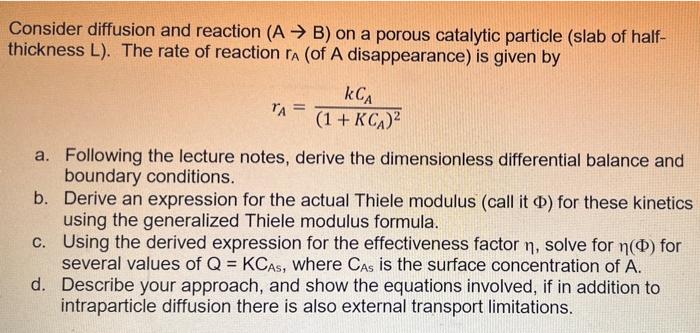
Consider diffusion and reaction (A B) on a porous catalytic particle (slab of half- thickness L). The rate of reaction ra (of A disappearance) is given by = kCA TA= (1 + KCA) a. Following the lecture notes, derive the dimensionless differential balance and boundary conditions. b. Derive an expression for the actual Thiele modulus (call it ) for these kinetics using the generalized Thiele modulus formula. c. Using the derived expression for the effectiveness factor n, solve for n(o) for several values of Q = KCAs, where Cas is the surface concentration of A. d. Describe your approach, and show the equations involved, if in addition to intraparticle diffusion there is also external transport limitations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


