Question: Consider laminar diffusion combustion shown in the figure below. Fuel and oxidizer, separated by a wall, diffuse into a hole in the wall and
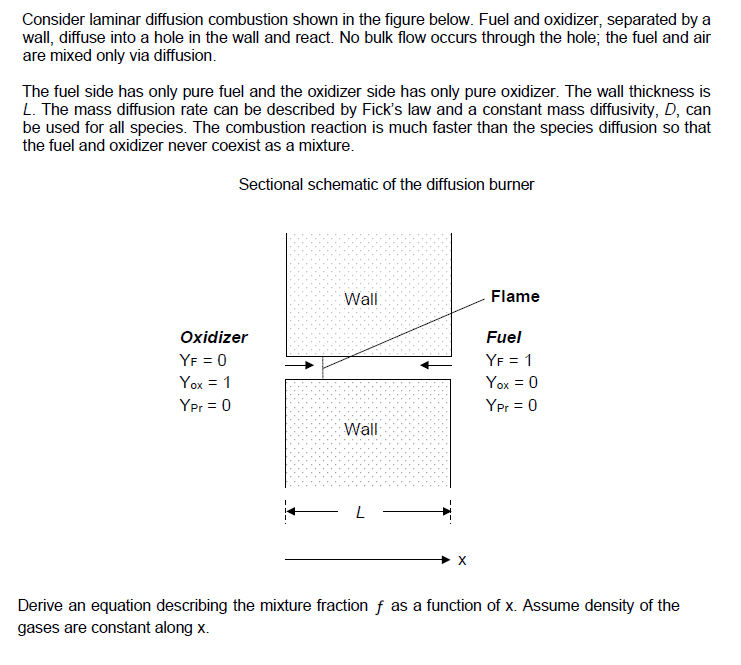
Consider laminar diffusion combustion shown in the figure below. Fuel and oxidizer, separated by a wall, diffuse into a hole in the wall and react. No bulk flow occurs through the hole; the fuel and air are mixed only via diffusion. The fuel side has only pure fuel and the oxidizer side has only pure oxidizer. The wall thickness is L. The mass diffusion rate can be described by Fick's law and a constant mass diffusivity, D, can be used for all species. The combustion reaction is much faster than the species diffusion so that the fuel and oxidizer never coexist as a mixture. Sectional schematic of the diffusion burner Wall Flame Oxidizer Fuel YF = 0 YF = 1 Yox = 1 Yox = 0 Ypr 0 = Ypr = 0 Wall: L Derive an equation describing the mixture fraction f as a function of x. Assume density of the gases are constant along x.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


