Question: Consider molecules with the molecular formula C4H8 0. (Select all that apply.) a. Choose the structural formula for a molecule with this molecular formula

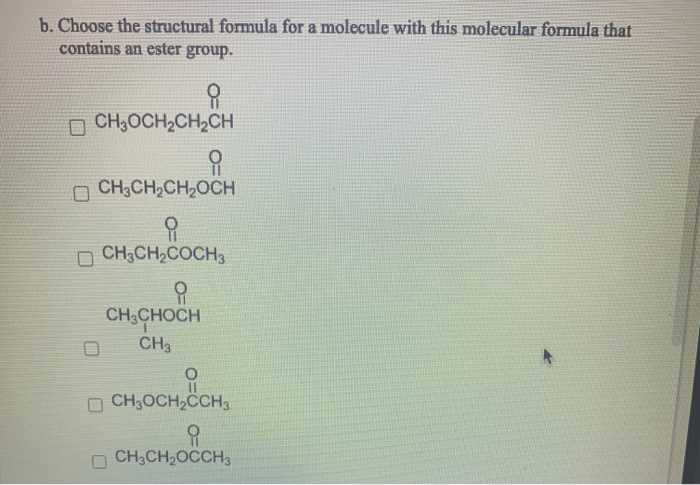
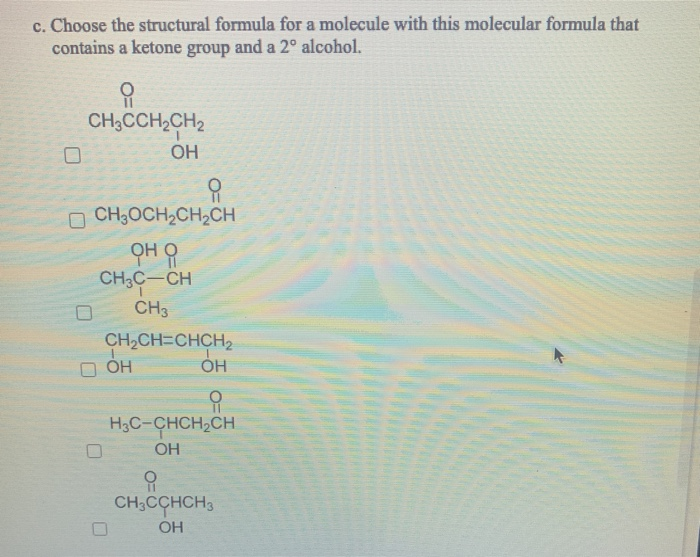
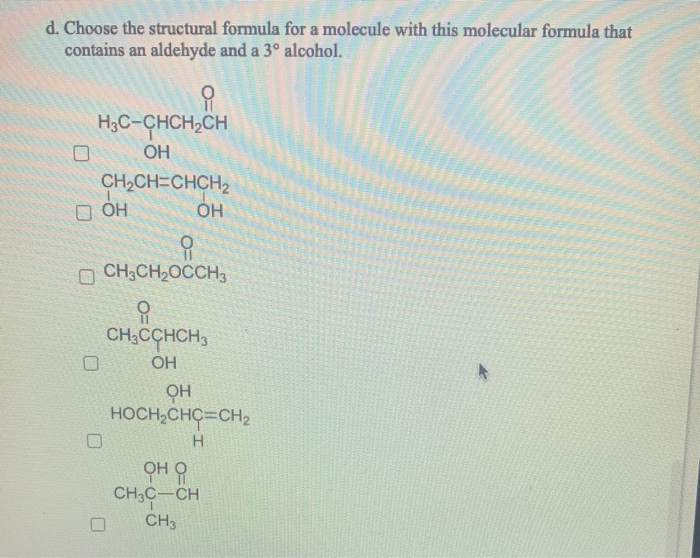
Consider molecules with the molecular formula C4H8 0. (Select all that apply.) a. Choose the structural formula for a molecule with this molecular formula that contains a carboxyl group. CH3CHCOH CH3 O CHCHCH3 OCHOCHCHCH CH3CHCOCH 3 CH3CHOCCH3 OCHCHCHCOH b. Choose the structural formula for a molecule with this molecular formula that contains an ester group. CH3OCH2CH2CH CH3CH2CH2OCH CH3CH2COCH3 CH3CHOCH CH3 CH3OCH2CCH3 CH3CH2OCCH3 c. Choose the structural formula for a molecule with this molecular formula that contains a ketone group and a 2 alcohol. CH3CCHCH2 OH CH3OCHCHCH CH3C-CH CH3 CHCH=CHCH OH H3C-CHCHCH CH3CCHCH3 OH d. Choose the structural formula for a molecule with this molecular formula that contains an aldehyde and a 3 alcohol. H3C-CHCHCH OH CHCH=CHCH D OH OH CH3CHOCCH3 CH3CCHCH3 OH OH HOCHCHC=CH H CH3C-CH CH3
Step by Step Solution
3.40 Rating (153 Votes )
There are 3 Steps involved in it
A O 50 4 b c CH3 CH CHC... View full answer

Get step-by-step solutions from verified subject matter experts


