Question: Consider the absorber - stripper system illustrated on the next page. The feed containing 3 0 % C O 2 , 1 0 % H
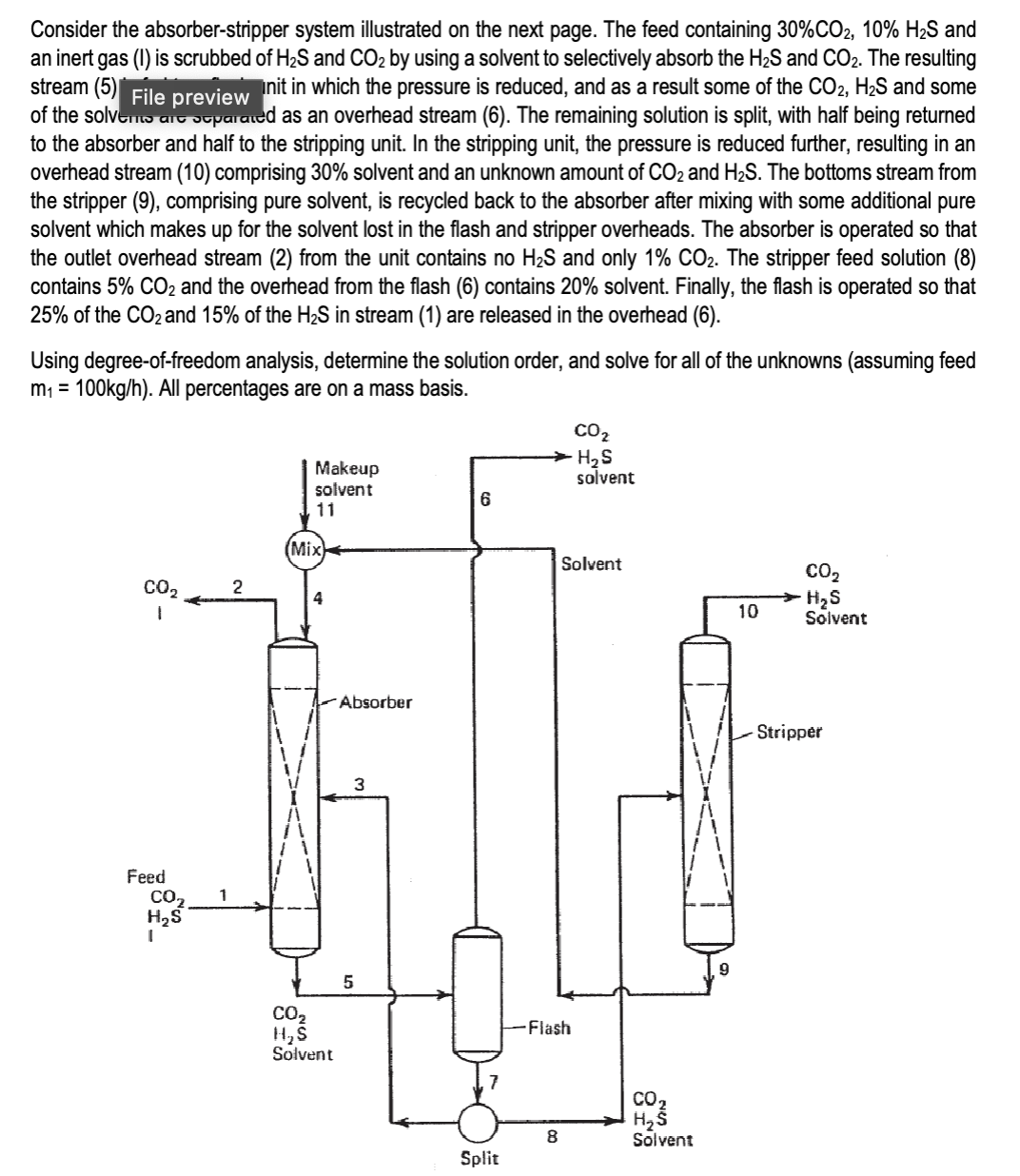
Consider the absorberstripper system illustrated on the next page. The feed containing and
an inert gas is scrubbed of and by using a solvent to selectively absorb the and The resulting
stream in which the pressure is reduced, and as a result some of the and some
of the solvers ar soparaded as an overhead stream The remaining solution is split, with half being returned
to the absorber and half to the stripping unit. In the stripping unit, the pressure is reduced further, resulting in an
overhead stream comprising solvent and an unknown amount of and The bottoms stream from
the stripper comprising pure solvent, is recycled back to the absorber after mixing with some additional pure
solvent which makes up for the solvent lost in the flash and stripper overheads. The absorber is operated so that
the outlet overhead stream from the unit contains no and only The stripper feed solution
contains and the overhead from the flash contains solvent. Finally, the flash is operated so that
of the and of the in stream are released in the overhead
Using degreeoffreedom analysis, determine the solution order, and solve for all of the unknowns assuming feed
: All percentages are on a mass basis.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


