Question: Consider the below incomplete Lewis Structure with missing atoms X,Y, and Z which are all elements in the second row of the periodic table of
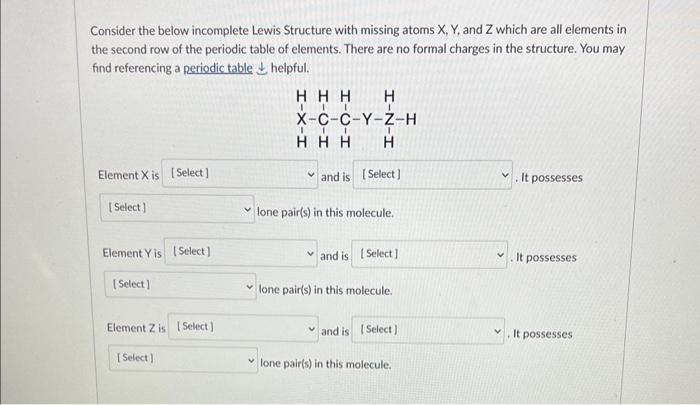
Consider the below incomplete Lewis Structure with missing atoms X,Y, and Z which are all elements in the second row of the periodic table of elements. There are no formal charges in the structure. You may find referencing a periodic table helpful. Element X is and is It possesses lone pair(s) in this molecule. Element Y is and is It possesses lone pair(s) in this molecule. Element Z is and is It possesses lone pair(s) in this molecule
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


