Question: Consider the case of a cube-shaped solid forming heterogeneously at a 90 edge of the container such that two sides of the cube-nucleus are in
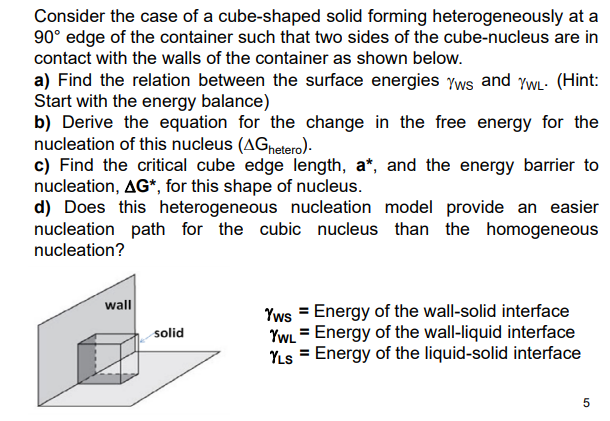
Consider the case of a cube-shaped solid forming heterogeneously at a 90 edge of the container such that two sides of the cube-nucleus are in contact with the walls of the container as shown below. a) Find the relation between the surface energies Ws and WL. (Hint: Start with the energy balance) b) Derive the equation for the change in the free energy for the nucleation of this nucleus (Ghetero). c) Find the critical cube edge length, a, and the energy barrier to nucleation, G, for this shape of nucleus. d) Does this heterogeneous nucleation model provide an easier nucleation path for the cubic nucleus than the homogeneous nucleation? wsWLLS=Energyofthewall-solidinterface=Energyofthewall-liquidinterface=Energyoftheliquid-solidinterface
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


