Question: Consider the Cu-Ag phase diagram provided. What phases are present at equilibrium if an alloy of Cu-50wt%Ag is held at 800C. What are the
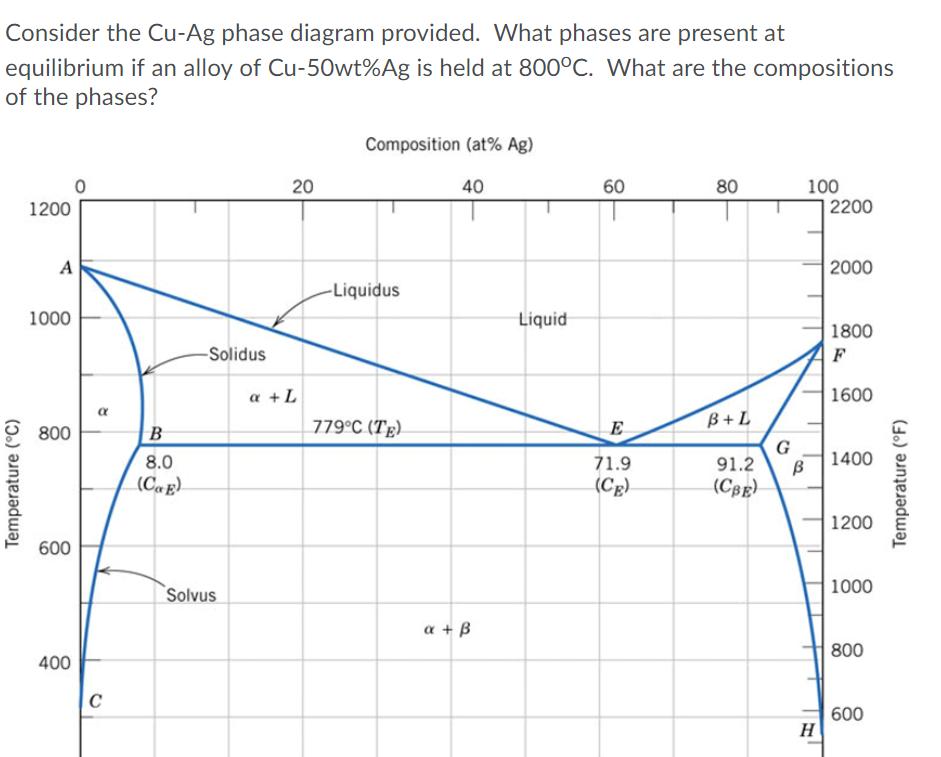

Consider the Cu-Ag phase diagram provided. What phases are present at equilibrium if an alloy of Cu-50wt%Ag is held at 800C. What are the compositions of the phases? Composition (at% Ag) 40 60 80 100 2200 1200 2000 -Liquidus 1000 Liquid 1800 -Solidus a +L 1600 779C (Tg). B+L 800 G 91.2 8.0 71.9 1400 (CE). (Cg) () 1200 600 1000 Solvus a + B 800 400 600 Temperature (C) 20 Temperature (F) The phases present are a (8 wt%Ag, 92 wt%Cu) and L (67 wt%Ag, 33 wt%Cu). Only x (50 wt%Ag, 50 wt%Cu) is present. The phases present are a (33 wt%Ag, 67wt%Cu) and L (67 wt%Ag, 33wt%Cu). The phases present are x (8 wt%Ag, 92 wt%Cu) and B (93 wt%Ag, 7 wt%Cu).
Step by Step Solution
3.41 Rating (160 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


