Question: Consider the data in the table for the reaction at 45 'C in carbon tetrachloride solution. IN 0,1 (M) 0 2.375 195 2.101 486 1.749
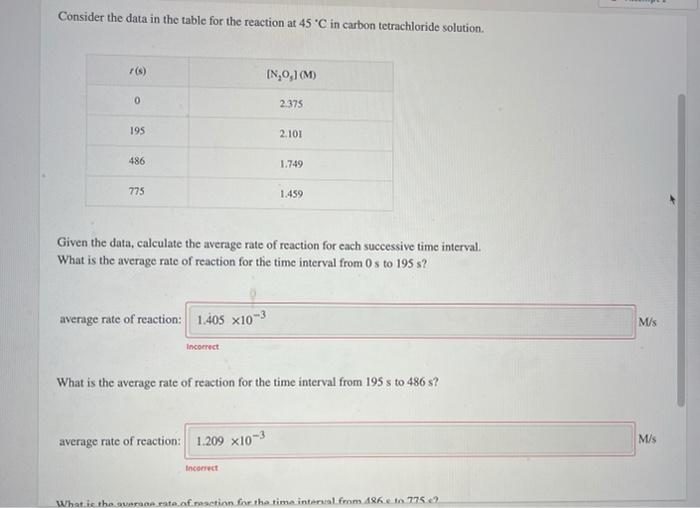

Consider the data in the table for the reaction at 45 'C in carbon tetrachloride solution. IN 0,1 (M) 0 2.375 195 2.101 486 1.749 775 1.459 Given the data, calculate the average rate of reaction for each successive time interval. What is the average rate of reaction for the time interval from 0s to 195 s? average rate of reaction: 1.405 x103 M/s Incorrect What is the average rate of reaction for the time interval from 195 s to 486 s? average rate of reaction: 1.209 x10-3 M/S Incorrect What is the wront of action for the time internal from 86 et 7752 Given the data, calculate the average rate of reaction for each successive time interval. What is the average rate of reaction for the time interval from 0 to 195 s? average rate of reaction: 1.405 x10-3 M/s Incorrect What is the average rate of reaction for the time interval from 195 s to 486 s? average rate of reaction: 1.209 x10-3 M/S Incorrect What is the average rate of reaction for the time interval from 486 s to 775 s? M/S average rate of reaction: 1.003 x10-3 Incorrect
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


