Question: Consider the equilibrium: A(g)+B(g)C (g)+2D(g). In one experiment, a mixture is prepared with the following initial concentrations: [A]0=0.2M,[B]0=0.07M, [C]0=1.3M,[D]0=0.05M. predict in which direction the reaction
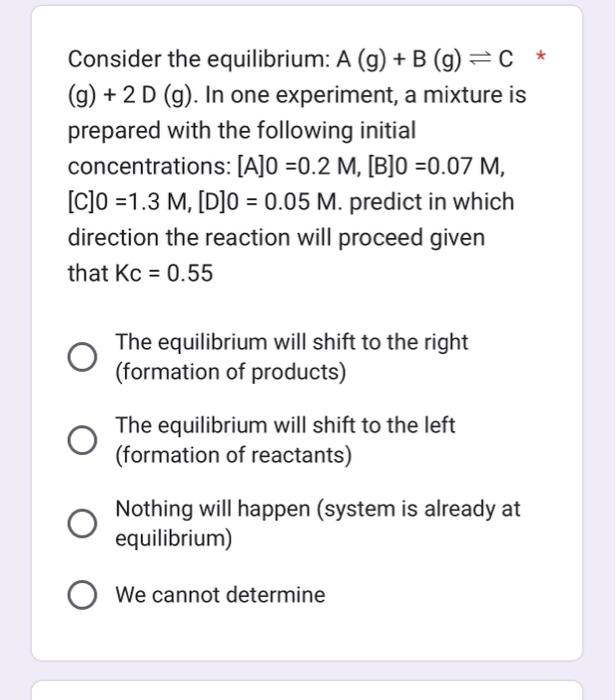
Consider the equilibrium: A(g)+B(g)C (g)+2D(g). In one experiment, a mixture is prepared with the following initial concentrations: [A]0=0.2M,[B]0=0.07M, [C]0=1.3M,[D]0=0.05M. predict in which direction the reaction will proceed given that Kc=0.55 The equilibrium will shift to the right (formation of products) The equilibrium will shift to the left (formation of reactants) Nothing will happen (system is already at equilibrium) We cannot determine
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


