Question: Consider the experimental data below for the decomposition of dinitrogen tetroxide at 100C. N2O4(g)2NO2(g) What is the equilibrium constant, Kc, for this set of experiments?
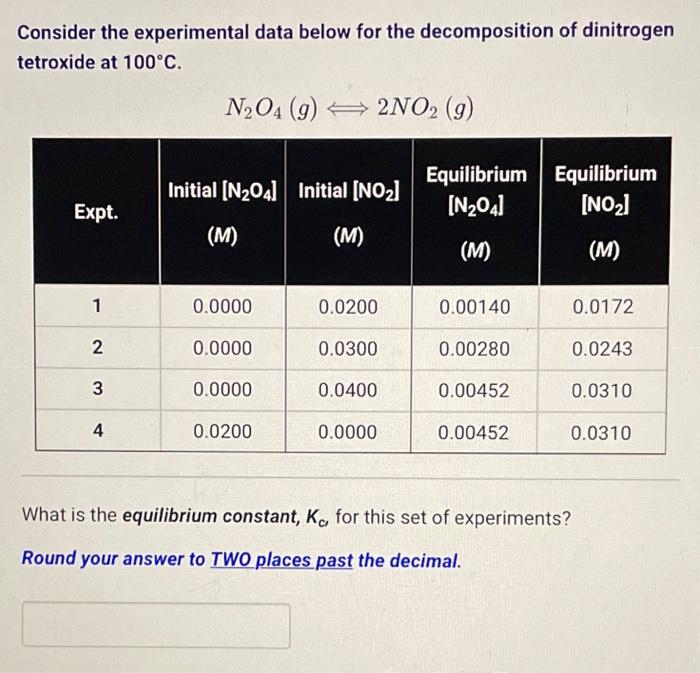
Consider the experimental data below for the decomposition of dinitrogen tetroxide at 100C. N2O4(g)2NO2(g) What is the equilibrium constant, Kc, for this set of experiments? Round your answer to TWO places past the decimal
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


