Question: Consider the following enzymic reaction E + S -> P + E Where S is the substrate and P is the product The reaction takes
Consider the following enzymic reaction
E + S -> P + E
Where S is the substrate and P is the product
The reaction takes place in 3 steps
E + S E*S
E*S + S S*E*S
E*S -> E + P
1) Derive the rate equation for substrate consumption (-r(S)) in terms of the concentration of the substrate. Utilize pseudo-steady-state approximation (PSSA) for the enzyme-substrate complex. Note that the rate equation should not include concentrations of the intermediates.
2) Explore the behavior of -r(S) at small and large concentrations of S. Quantitavily show that the equation you found in part 1 can produce a plot similar to the one below.
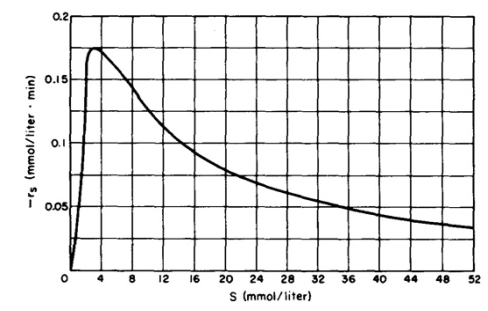
3) Derive an expression for the maximum of -r(S)
0.21 0.15 -Is (mmol/liter . min) 0.1 0.05) o 12 16 24 36 40 48 52 20 28 32 S (mmol/liter)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


