Question: Consider the following equilibrium system: C O ( g ) + H 2 O ( g ) C O 2 ( g ) + H
Consider the following equilibrium system:
The equilibrium concentrations of a particular mixture in a reaction vessel are:
and
How many moles of must now be injected in order to create a new equilibrium where the
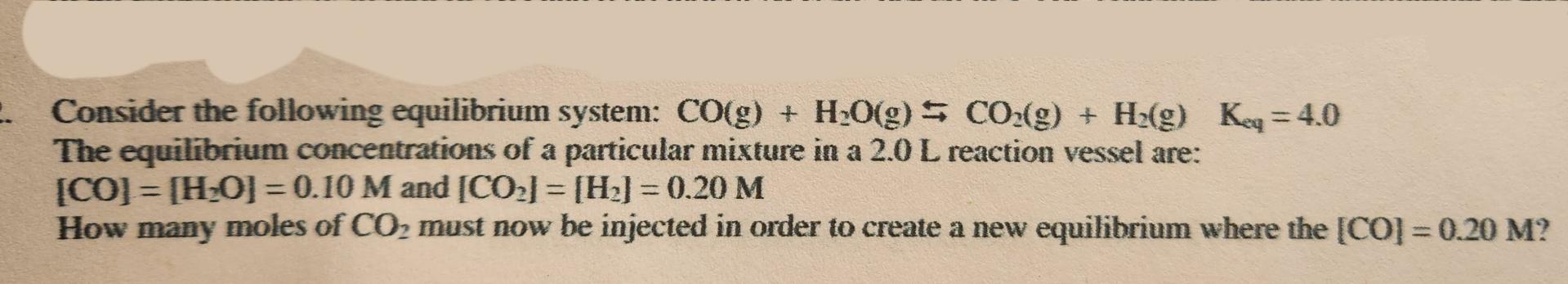
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


