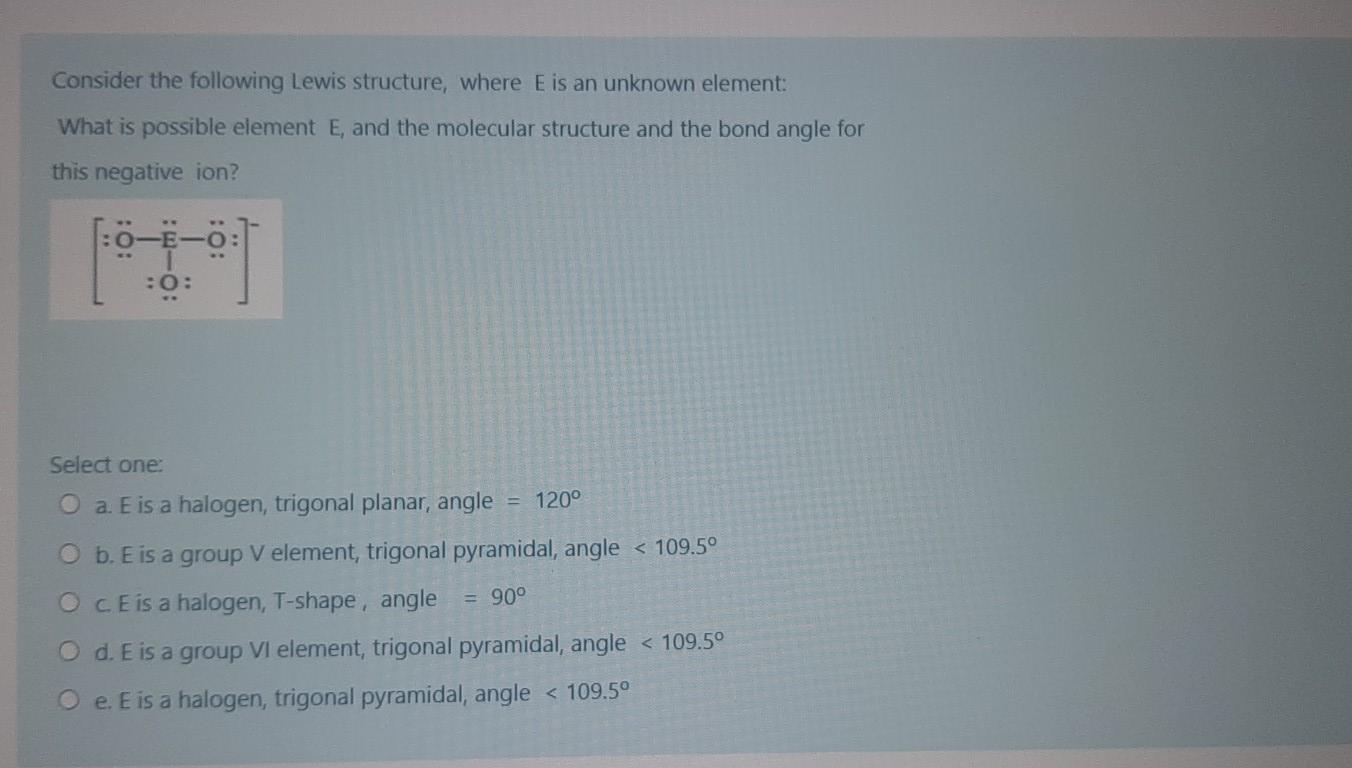
Question: Consider the following Lewis structure, where E is an unknown element: What is possible element E, and the molecular structure and the bond angle
Consider the following Lewis structure, where E is an unknown element: What is possible element E, and the molecular structure and the bond angle for this negative ion? :0: Select one: O a. E is a halogen, trigonal planar, angle = 120 O b. E is a group V element, trigonal pyramidal, angle < 109.5 O CE is a halogen, T-shape, angle = 90 O d. E is a group VI element, trigonal pyramidal, angle < 109.5 O e. E is a halogen, trigonal pyramidal, angle < 109.5
Step by Step Solution
3.39 Rating (165 Votes )
There are 3 Steps involved in it
As we know the valence electron on the oxygen atom is 6 But in the question the normal o... View full answer

Get step-by-step solutions from verified subject matter experts