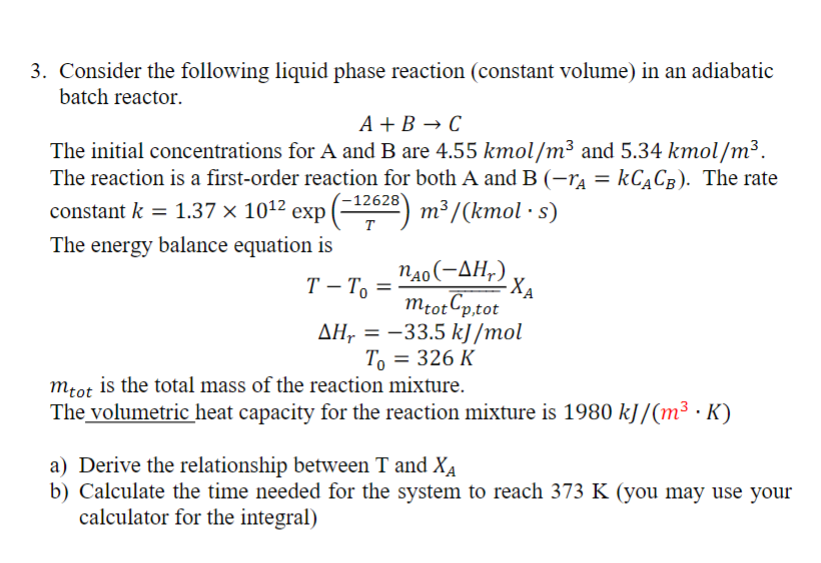
Question: Consider the following liquid phase reaction ( constant volume ) in an adiabatic batch reactor. A + B C The initial concentrations for A and
Consider the following liquid phase reaction constant volume in an adiabatic
batch reactor.
The initial concentrations for A and are kmo and kmo
The reaction is a firstorder reaction for both A and The rate
constant exp
The energy balance equation is
is the total mass of the reaction mixture.
The volumetric heat capacity for the reaction mixture is
a Derive the relationship between and
b Calculate the time needed for the system to reach you may use your
calculator for the integralConsider the following liquid phase reaction constant volume in an adiabatic
batch reactor.
The initial concentrations for A and B are and
The reaction is a firstorder reaction for both A and B
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


