Question: Consider the following reaction at equilibrium. What effect will reducing the volume of the reaction mixture have on the system? CuS(s)+O2(s)Cu(s)+SO2(s) The equilibrium constant will

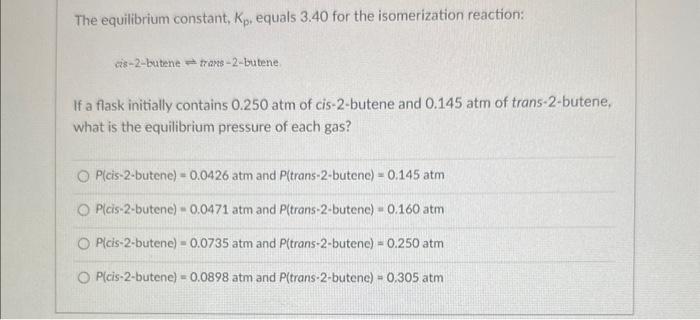
Consider the following reaction at equilibrium. What effect will reducing the volume of the reaction mixture have on the system? CuS(s)+O2(s)Cu(s)+SO2(s) The equilibrium constant will decrease. No effect will be observed. The reaction will shift to the right in the direction of products. The equilibrium constant will increase. The reaction will shift to the left in the direction of reactants. The equilibrium constant, Kp, equals 3.40 for the isomerization reaction: ris-2-butenetrans-2-butene If a flask initially contains 0.250 atm of cis-2-butene and 0.145 atm of trans-2-butene, what is the equilibrium pressure of each gas? P( cis-2-butene )=0.0426atm and P( trans-2-butene )=0.145atm P( cis-2-butene )=0.0471atm and P( trans-2-butene )=0.160atm P( cis-2-butene )=0.0735atm and P( trans-2-butene )=0.250atm P( cis-2-butene )=0.0898atm and P( trans-2-butene )=0.305atm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


