Question: Consider the following two reactions and the corresponding enthalpy changes: CH4(8) +2 O2(g) CO2(g) +2 H20(1), AH1 2 CH4(8) + O2(g) 2 CH3OH(1), AH2 )
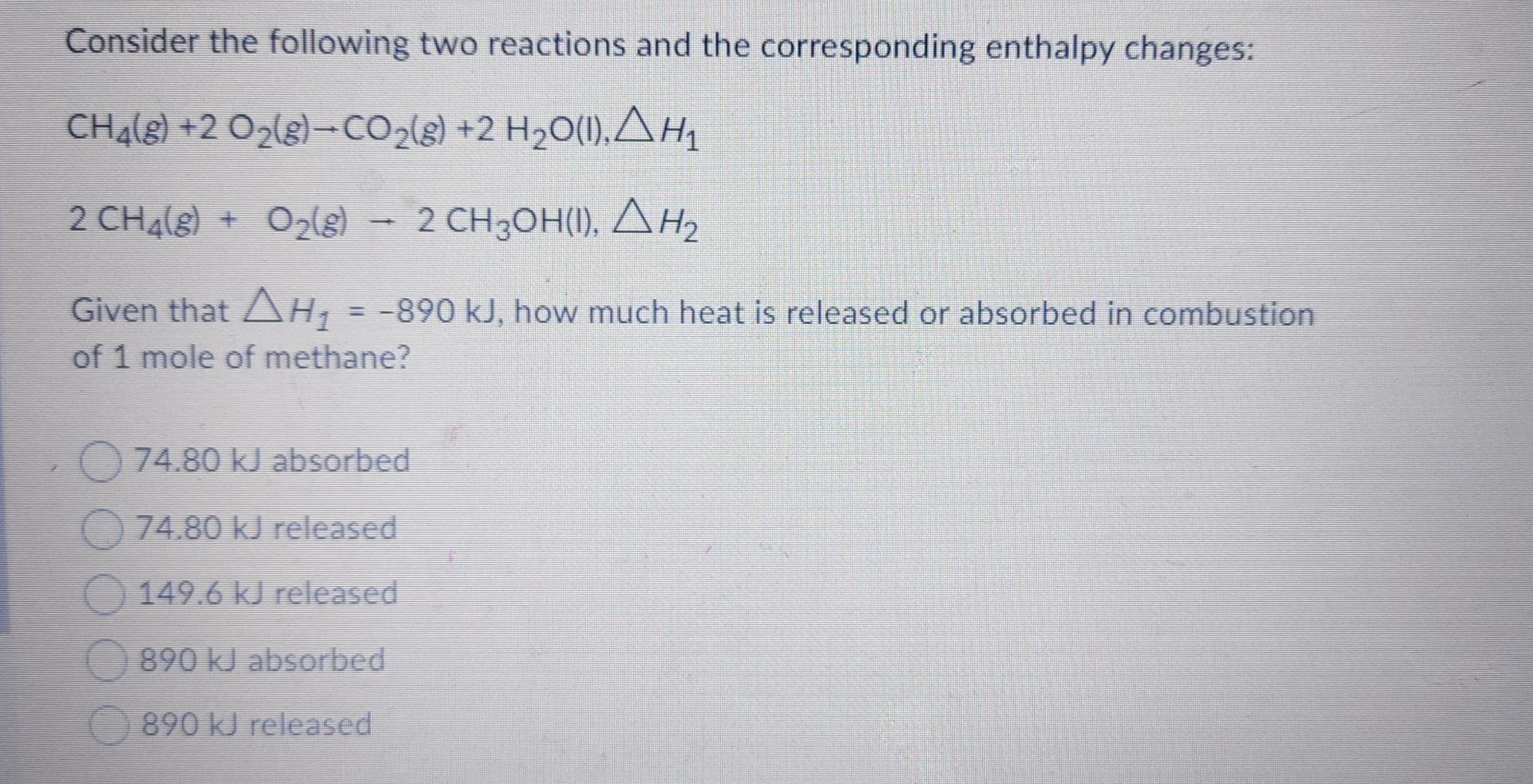

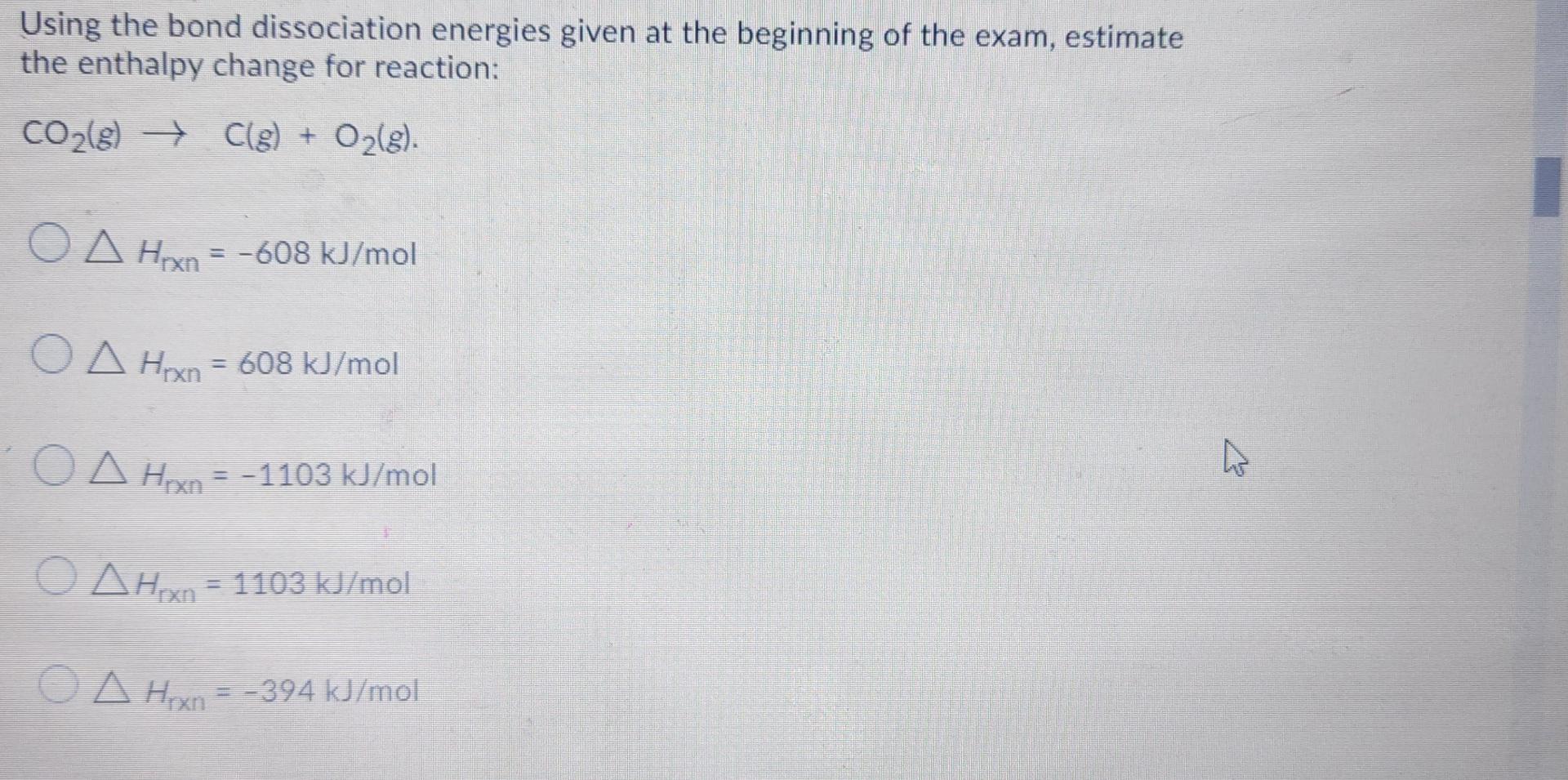


Consider the following two reactions and the corresponding enthalpy changes: CH4(8) +2 O2(g) CO2(g) +2 H20(1), AH1 2 CH4(8) + O2(g) 2 CH3OH(1), AH2 ) ), Given that AH2 = -890 kJ, how much heat is released or absorbed in combustion of 1 mole of methane? 74.80 kJ absorbed 74.80 kJ released 149.6 kJ released 890 kJ absorbed 890 kJ released Consider the following two reactions and the corresponding enthalpy changes: CH4(g) +2 O2(g)-CO2(g) +2 H30(I),, 2 CH4(g) + O2(g) 2 CHOH(1), , Using the heats of formation given at the beginning of the exam, determine the enthalpy change for the second reaction, AH , = -163.8k , = -327.6k , = 163.8kg , = 327.6k , = Using the bond dissociation energies given at the beginning of the exam, estimate the enthalpy change for reaction: CO2(8) Cle) + O2(g). O A Hex = -608 kJ/mol O A Han = 608 kJ/mol O A HxA = -1103 kJ/mol o Ahon = 1103 kJ/mol o A Hexn A Hoxn=-394 kJ/mol Standard enthalpy of formation of N(g) is 472 kJ/mol. From this information, estimate the bond enthalpy of N2. 236 kJ/mol 326 kJ/mol 944 kJ/mol 472 kJ/mol 163 kJ/mol The solubility of gases in water depends on temperature. Which body of water do you expect to have a lower concentration of N2 dissolved in it: The warm Caribbean Sea or the cold Arctic Ocean? The Arctic Ocean The Caribbean Sea Not enough info given Since the atmospheric pressure is the same, they will have equal concentrations of N2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


