Question: Consider the mechanism. Step 1: 2 AB equilibrium Step 2: B + CD slow Overall: 2 A + CD Determine the rate law for the
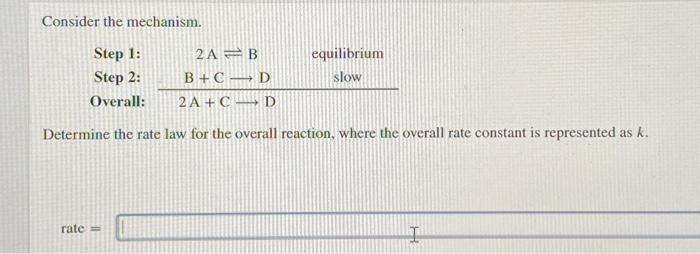
Consider the mechanism. Step 1: 2 AB equilibrium Step 2: B + CD slow Overall: 2 A + CD Determine the rate law for the overall reaction, where the overall rate constant is represented as k. rate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


