Question: Consider the overall reaction: A + B + C + D + 2 E. A set of kinetics experiments were performed, and the data is
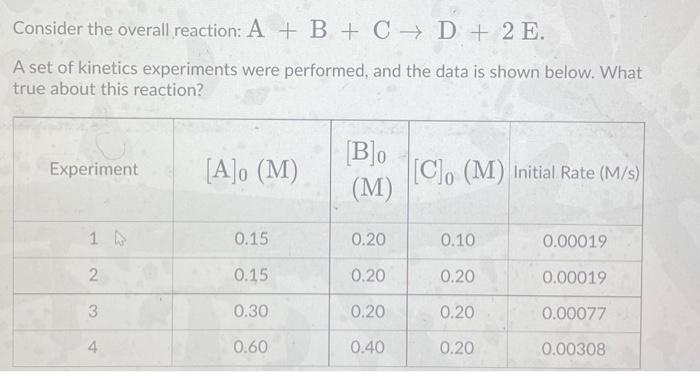

Consider the overall reaction: A + B + C + D + 2 E. A set of kinetics experiments were performed, and the data is shown below. What true about this reaction? Experiment [A]o (M) [B] (M) [C]. (M) initial Rate (M/s) 1 W 0.15 0.20 0.10 0.00019 2. 0.15 0.20 0.20 0.00019 3 0.30 0.20 0.20 0.00077 0.60 0.40 0.20 0.00308 This reaction is zeroth order overall, and none of the reactants are involved in the rate-determining step. This reaction is second order overall, and both A and B are involved in the rate- determining step. This reaction is second order overall, and reactant A is involved in the rate- determining step. This reaction is first order overall, and reactant B is involved in the rate- determining step. This reaction is third order overall, and all three reactants are involved in the rate-determining step
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


