Question: Consider the reaction 12(9) + Cl2(9)210 (9) Using the standard thermodynamic data in the tables linked above, calculate the equilibrium constant for this reaction at

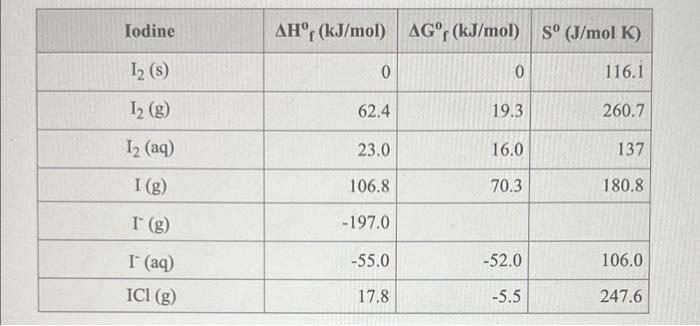
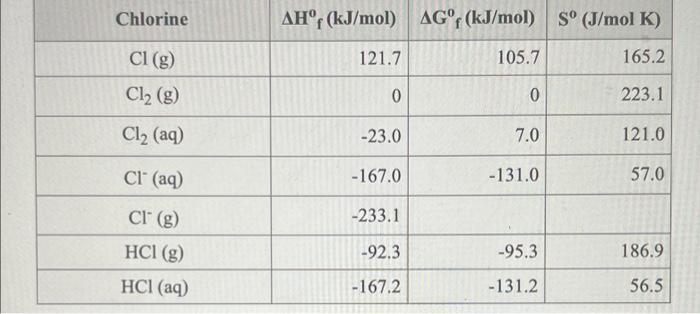
Consider the reaction 12(9) + Cl2(9)210 (9) Using the standard thermodynamic data in the tables linked above, calculate the equilibrium constant for this reaction at 298.15K. ANSWER: Iodine AHr (kJ/mol) AGr (kJ/mol) S (J/mol K) 12 (5) 0 0 116.1 12 (g) 62.4 19.3 260.7 12 (aq) 23.0 16.0 137 I(g) 106.8 70.3 180.8 1 (8) -197.0 -55.0 -52.0 106.0 I (aq) ICI (g) 17.8 -5.5 247.6 Chlorine AHF (kJ/mol) AGf (kJ/mol) S (J/mol K) Cl (g) 121.7 105.7 165.2 0 0 223.1 Cl2 (g) Cl2 (aq) -23.0 7.0 121.0 Cl(aq) -167.0 -131.0 57.0 Cl' (g) -233.1 -92.3 -95.3 186.9 HCI (g) HCl(aq) - 167.2 -131.2 56.5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


