Question: Consider the reaction 2B ---> C + 3D This reaction is carried out at two different temperatures and the following two values of the rate
Consider the reaction 2B ---> C + 3D This reaction is carried out at two different temperatures and the following two values of the rate law constant are observed: 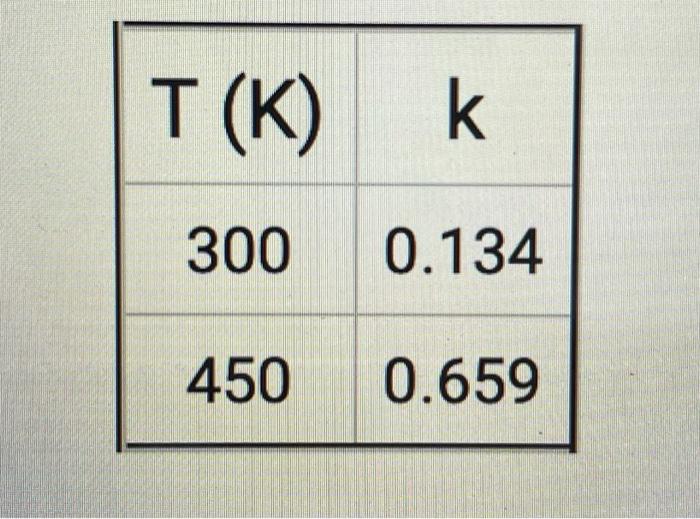
Calculate the activation energy values (kJ/mol) of this reaction and the value of A, the pre-exponential factor of the Arrhenius equation.

T(K) k 300 0.134 450 0.659
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


