Question: Consider the reaction: 2NO(9) + Br (9) = 2BrNO(9) K = 32.9 at 315 K In a reaction mixture at equilibrium the partial pressure of
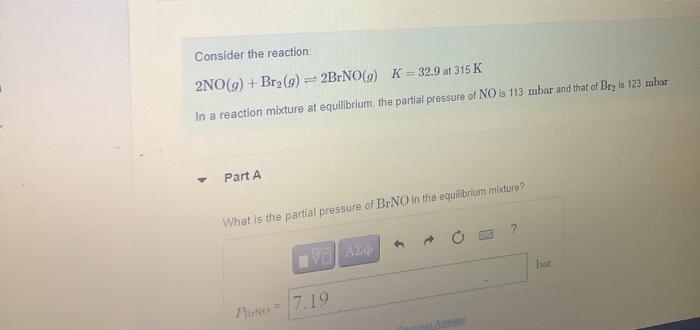
Consider the reaction: 2NO(9) + Br (9) = 2BrNO(9) K = 32.9 at 315 K In a reaction mixture at equilibrium the partial pressure of NO is 113 mbar and that of Bryls 123mbur Part A What is the partial pressure of BINO in the equilibrium mixture? VOA24 har Phone = 7.19
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


