Question: Consider the reaction: C O 2 + 4 H C H 2 O + H 2 O Suppose 1 . 8 1 gCO 2 and
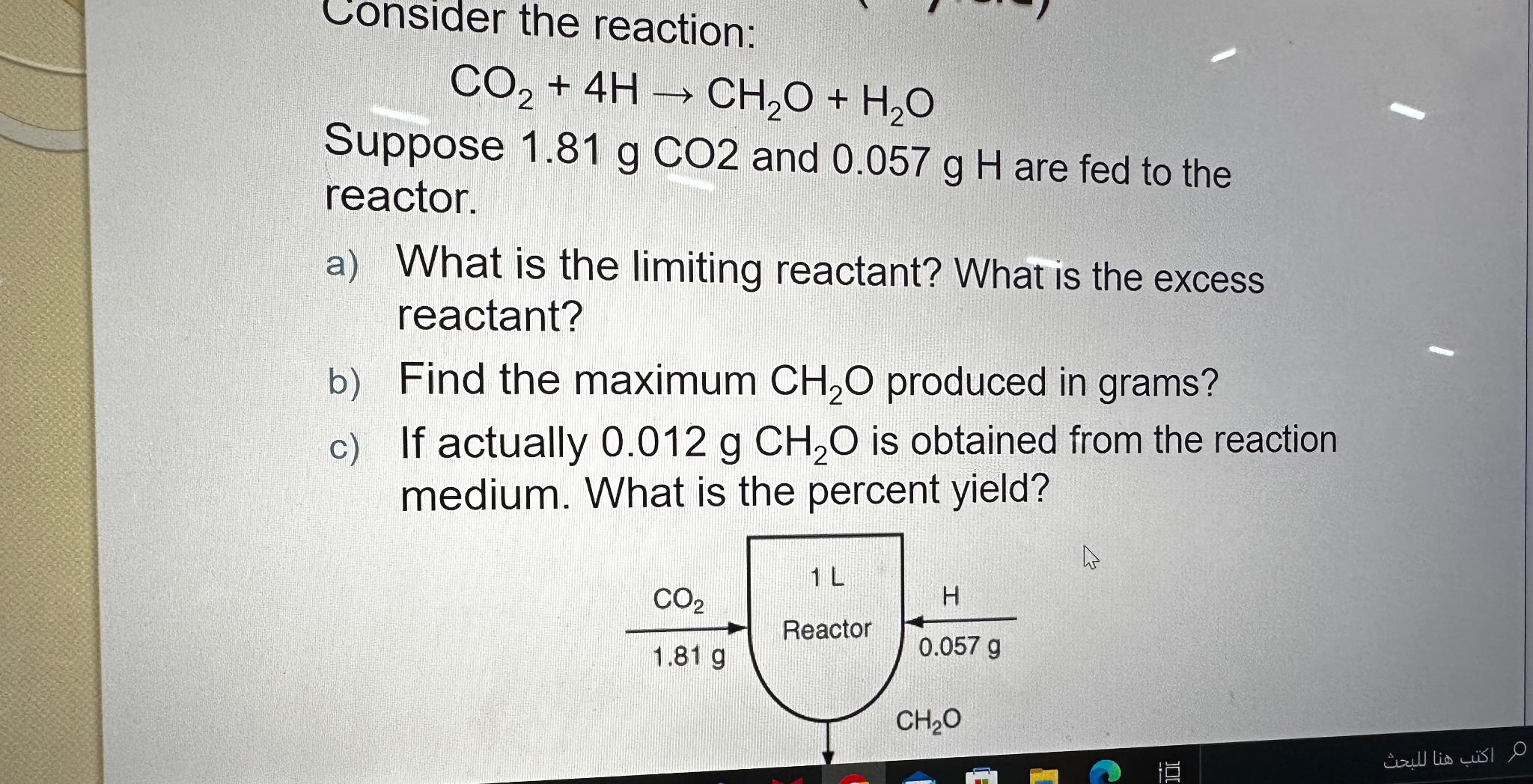
Consider the reaction:
Suppose gCO and are fed to the reactor.
a What is the limiting reactant? What is the excess reactant?
b Find the maximum produced in grams?
c If actually is obtained from the reaction medium. What is the percent yield?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


